In recent years,the synthesis of quinazolines and its derivatives has become a hot spot in organic synthetic chemistry due to their broad biological and medicinal activities,such as antibacterial,anticarcinogenic and antihypertensive properties[1, 2, 3, 4, 5].Usually,the traditional synthesis of quinazolines involves reactions of Bischler cyclization,dicarbonyl compounds with diamines and reactions from 2-aminobenzonitriles or anthranilic acids as well as N-arylbenzamides[6, 7, 8, 9].Our group have been focusing in the synthesis of quinazolines and a variety of excellent approaches to the quinazolines were developed[10, 11, 12, 13].At the same time,other groups also developed some novel methods to prepare these quinazoline derivatives[14, 15, 16, 17, 18, 19, 20].For example,Li[20]developed a KI-catalyzed synthesis of quinazolines from 2-aminobenzoketones,toluene and ammonium salt.In these syntheses,the key step is to construct C-N bonds of the cyclization.Recently,transitionmetal-catalyzed oxidative aminations of sp3 C-H bond have emerged as important methods for C-N bond formations because of short steps and atom-economical advantages[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38].In particular,copper as an inexpensive and lowly toxic metal catalyst,has been employed to catalyze the formation of C-N bond via a sp3 C-H amination[22, 39, 40, 41, 42, 43, 44, 45, 46, 47].For instance,copper-catalyzed cascade coupling of 2-halobenzaldehyde with acetamidine hydrochloride (or benzaldehyde) to construct C-N bond was reported[48, 49, 50, 51, 52].Nevertheless,these methods generally suffered from limitations of substrate generality and availability of starting material.Especially,for those substrates bearing electro-withdrawing group,there reaction hardly occurs.Therefore,to develop some novel and efficient method for the synthesis of quinazolines still remains highly desirable.
Herein,we report a novel copper-catalyzed double oxidative C-H aminations of methylarenes with 2-aminobenzoketones and ammonium acetate,constructing one C55N bond and one C-N bond in one step.
2.ExperimentalUnless otherwise indicated,all commercial reagents and solvent were used without additional purification.1H NMR spectra were recorded with a Bruker AVIII-400 spectrometer.Chemical shifts (in ppm) were referenced to tetramethylsilane (δ=0) in CDCl3 as internal standard.13C spectra were obtained by the same NMR spectrometer and were calibrated with CDCl3(δ=77.00).HRMS (ESI) were recorded on a WatersTM Q-TOF Premier Mass Spectrometer.
2.1.Preparation of substrates Substrates 1a,1f,1m and 1n are commercially available.Other substrates (1b-1e,1g-1l and 1o) were prepared using our previous literature procedure[10]. 2.2.Experimental Procedure for preparation of 3Substrate 1(0.2 mmol),NH4OAc (31.2 mg,0.4 mmol),CuCl2·2H2O (6.8 mg,20 mol%),TBHP (90 mL,70% aq,0.6 mmol),were added to a tube,followed by addition of solvent 2(2 mL).The mixture was stirred at assigned temperature and monitored by TLC.The solution was cooled to r.t.,diluted with ethyl acetate (5 mL),washed with saturated aqueous sodium hydrogen sulfite.The aqueous layers was extracted with EtOAc (3× 10 mL),the combined organic layers were dried over Na2SO4,filtered,and evaporated under vacuum.The residue was purified by column chromatography on silica gel (petroleum ether:ethyl acetate=20:1) to afford the desired product 3.
Characterization data of compounds 3 were given in Supporting information.
3.Results and discussionWe began our studies with the reaction of (2-amino-phenyl)-phenyl-methanone (1a,1 equiv.),NH4OAc (2 equiv.),tert-butyl hydroperoxide (TBHP,70% in water,2 equiv.) as an oxidant,20 mol% Cu(OAc)2 as catalyst and 2 mL toluene (2a) as the solvent and reagent.When heated under air at 80 ℃ overnight,2,4-diphenyl quinazoline (3aa) was obtained in 43% yield (Table 1,entry 1).When we replaced Cu(OAc)2 with other transition metal acetates,the reaction yield was reduced (Table 1,entries 2-4).Among various copper salts examined (Table 1,entries 5-9),copper chloride dehydrate gave the best yield of 86%(Table 1,entry 6).Next,we optimized the oxidant such as di-tert-butyl peroxide (DTBP),cumene hydroperoxide (CHP),H2O2(30% in water) and O2(Table 1,entries 10-13).Also,the nitrogen sources (Table 1,entries 14-16) and the reaction temperature (Table 1,entries 17-19) were optimized,but no better yield was obtained.In addition,we increased the loading of TBHP to 3 equiv.since 1a was not used out,giving 3aa in 88% yield (Table 1,entry 20).Finally,the optimal conditions were described in entry 20.
| Table 1 Optimization of reaction conditions.a |
Subsequently,we investigated the substrate scope of this reaction under the optimized reaction conditions and obtained the product (3aa-3oa,Fig.1).Firstly,when R1 is an aromatic substituent,the reaction of substrates 1a-1d can be carried out to give the corresponding products 3aa-3da with good yields.Substrates with electro-withdrawing group (4-F and 4-Br) gave higher yields than substrates with electro-donating group (4-Me) on the phenyl ring.When R1 is a 2-naphthyl substituent,the corresponding product 3ea was generated with an 88% yield.To our delight,substrate 1f-1l with aliphatic substituents also gave the corresponding products 3fa-3la in good yields.When R1 is an aliphatic alkyl group,the alkyl with the tertiary carbon favoured the reaction,as shown in 3la.Notably,it was found that R1 benzylic C-H can be oxidized into C=O bond to give 3ga'.On the other hand,R2 substituent had a little influence on the reaction.When R2 alternated from electroN-donation group (5-Me) to electro-withdrawing group (5-Cl and 5-NO2),the yields was reduced to some extent,as shown in 3ma,3na and 3oa.
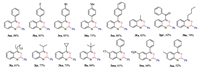
|
Download:
|
| Fig. 1.Structures and yields of compounds 3aa–3oa. | |
Then we tried to use different methylarenes 2 as the solvent and regent to extend generality of this reaction (Table 2).Both methylarenes bearing electro-donating group (2b-2e) and weaker electro-withdrawing group (2f and 2g) could generate the desired products 3ab-3ag with good to excellent yields.The position of the methyl group on the phenyl ring of 2 affected the reaction yields slightly (Table 2,entries 1-3).However,when a strong electrowithdrawing-group was induced into ortho-position (2h),noproduct was detected (Table 2,entry 7),perhaps due to the strong electronic effect of the nitro group.
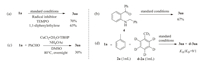
To gain an insight into the mechanism,several control experiments were carried out.Firstly,it was observed that the reaction was not obviously inhibited in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or 1,1-diphenylethlene.No benzyl radical was obtained by EPR experiment[53, 54].Two results suggested that the reaction did not undergo a radical pathway (Scheme 1a).When (2-benzylamino-phenyl)-(phenyl)-methanone 4 was employed as a substrate to carry out the reaction under standard conditions,67% of 3aa was obtained.This indicated that 4 may be the intermediate of the reaction (Scheme 1b).Moreover,trace amount of benzaldehyde could be detected in the model reaction.However,when 1 equiv.of benzaldehyde was used as substrate,only 30% yield of 3aa was obtained (Scheme 1c).This indicated that benzaldehyde may be not the intermediate of the reaction.Finally,a large intermolecular kinetics isotope effect (kH/kD=9) was observed by 1H NMR and HRMS from toluene and d8-toluene,which indicated that the C-H cleavage was a rate-determining step (Scheme 1d).
|
Download:
|
| Scheme. 1.Control experiment and KIE experiment. | |
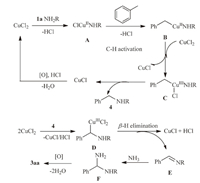
On the basis of the above results and the previous reports[22, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48],a plausible catalytic cycle of this transformation is proposed (Scheme 2).Initially,the coordination of 1a to one CuII species and subsequent ligand exchange generates the copper complex A,which forms the benzyl/CuII species B by benzylic C-H activation.Then the oxidation of B with another CuII species gives the benzyl/CuIII complex C and one CuI species.Reductive elimination of C gives the intermediate 4 and another CuI species.And 4 were converted into E with β-H elimination.Finally,E with NH3 were converted into F via a similar catalytic cycle,which forms 3aa through a condensation and oxidation.The generated CuI species is then oxidized to the CuII species by TBHP.
|
Download:
|
| Scheme. 2.A plausible catalytic cycle. | |
In summary,we developed a copper-catalyzed oxidative amination of benzylic C-H bonds of methylarenes with ammonia and 2-aminobenzoketones under mild conditions.By virtue of this method,a series of 2-arylquinazolines was efficiently synthesized in good yields.Copper-catalyzed oxidative C-H amination of methylarenes for the synthesis of other heterocycles is ongoing in our laboratory.
AcknowledgmentsWe are grateful to the National Nature Science Foundation of China (Nos.2127222,91213303,21172205,21432009,21472177).
Appendix A.Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.07.008.
| [1] | P.A. Plé, T.P. Green, L.F. Hennequin, et al., Discovery of a new class of anilinoquinazoline inhibitors with high affinity and specificity for the tyrosine kinase domain of c-Src, J. Med. Chem. 47(2004) 871-887. |
| [2] | L.A. Doyle, D.D. Ross, Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2), Oncogene 22(2003) 7340-7358. |
| [3] | K. Waisser, J. Gregor, H. Dostál, et al., Influence of the replacement of the oxo function with the thioxo group on the antimycobacterial activity of 3-aryl-6,8-dichloro-2H-1,3-benzoxazine-2,4(3H)-diones and 3-arylquinazoline-2,4(1H,3H)-diones, Farmaco 56(2001) 803-807. |
| [4] | J.P. Michael, Quinoline, quinazoline and acridone alkaloids, Nat. Prod. Rep. 25(2008) 166-187. |
| [5] | V. Colotta, D. Catarzi, F. Varano, et al., Structural investigation of the 7-chloro-3-hydroxy-1H-quinazoline-2,4-dione scaffold to obtain AMPA and kainate receptor selective antagonists. Synthesis, pharmacological, and molecular modeling studies, J. Med. Chem. 49(2006) 6015-6026. |
| [6] | A. Witt, J. Bergman, Recent developments in the field of quinazoline chemistry, Curr. Org. Chem. 7(2003) 659-677. |
| [7] | D.J. Connolly, D. Cusack, T.P. O'Sullivan, et al., Synthesis of quinazolinones and quinazolines, Tetrahedron 61(2005) 10153-10202. |
| [8] | L. Jiarong, C. Xian, S. Daxin, et al., A new and facile synthesis of quinazoline-2,4(1H,3H)-diones, Org. Lett. 11(2009) 1193-1196. |
| [9] | P.R. Marsham, A.L. Jackman, A.J. Barker, et al., Quinazoline antifolate thymidylate synthase inhibitors:replacement of glutamic acid in the C2-methyl series, J. Med. Chem. 38(1995) 994-1004. |
| [10] | J. Zhang, D. Zhu, C. Yu, et al., A simple and efficient approach to the synthesis of 2-phenylquinazolines via sp3 C-H functionalization, Org. Lett. 12(2010) 2841-2843. |
| [11] | J. Zhang, C. Yu, S. Wang, et al., A novel and efficient methodology for the construction of quinazolines based on supported copper oxide nanoparticles, Chem. Commun. 46(2010) 5244-5246. |
| [12] | Y. Yan, Z. Wang, Metal-free intramolecular oxidative decarboxylative amination of primary a-amino acids with product selectivity, Chem. Commun. 47(2011) 9513-9515. |
| [13] | Y. Yan, Y. Zhang, C. Feng, et al., Selective iodine-catalyzed intermolecular oxidative amination of C (sp3)-H bonds with ortho-carbonyl-substituted anilines to give quinazolines, Angew. Chem. Int. Ed. 51(2012) 8077-8081. |
| [14] | R. Sarma, D. Prajapati, Microwave-promoted efficient synthesis of dihydroquinazolines, Green Chem. 13(2011) 718-722. |
| [15] | Z.H. Zhang, X.N. Zhang, L.P. Mo, et al., Catalyst-free synthesis of quinazoline derivatives using low melting sugar-urea-salt mixture as a solvent, Green Chem. 14(2012) 1502-1506. |
| [16] | Z. Chen, J. Chen, M. Liu, et al., Unexpected copper-catalyzed cascade synthesis of quinazoline derivatives, J. Org. Chem. 78(2013) 11342-11348. |
| [17] | B. Han, C. Wang, R.F. Han, et al., Efficient aerobic oxidative synthesis of 2-aryl quinazolines via benzyl C-H bond amination catalyzed by 4-hydroxy-TEMPO, Chem. Commun. 47(2011) 7818-7820. |
| [18] | X. Su, C. Chen, Y. Wang, et al., One-pot synthesis of quinazoline derivatives via [2+2+2]cascade annulation of diaryliodonium salts and two nitriles, Chem. Commun. 49(2013) 6752-6754. |
| [19] | Y. Wang, X. Su, C. Chen, One-pot synthesis of multiply substituted quinoline and quinazoline derivatives via[2+2+2]cascade annulation with diaryliodonium salts, Synlett 24(2013) 2619-2623. |
| [20] | D. Zhao, Q. Shen, J.-X. Li, Potassium iodide-catalyzed three-component synthesis of 2-arylquinazolines via amination of benzylic C-H bonds of methylarenes, Adv. Synth. Catal. 357(2015) 339-344. |
| [21] | W.W. Sun, P. Cao, R.Q. Mei, et al., Palladium-catalyzed unactivated C (sp3)-H bond activation and intramolecular amination of carboxamides:a new approach to β-lactams, Org. Lett. 16(2013) 480-483. |
| [22] | R. Xia, H.Y. Niu, G.R. Qu, et al., CuI controlled C-C and C-N bond formation of heteroaromatics through C (sp3)-H activation, Org. Lett. 14(2012) 5546-5549. |
| [23] | H. Lu, Y. Hu, H. Jiang, et al., Stereoselective radical amination of electrondeficient C (sp3)-H bonds by Co(II)-based metalloradical catalysis:direct synthesis of a-amino acid derivatives via α-C-H amination, Org. Lett. 14(2012) 5158-5161. |
| [24] | H.J. Kim, J. Kim, S.H. Cho, et al., Intermolecular oxidative C-N bond formation under metal-free conditions:control of chemoselectivity between aryl sp2 and benzylic sp3 C-H bond imidation, J. Am. Chem. Soc. 133(2011) 16382-16385. |
| [25] | W.C. Gao, S. Jiang, R.L. Wang, et al., Iodine-mediated intramolecular amination of ketones:the synthesis of 2-acylindoles and 2-acylindolines by tuning N-protecting groups, Chem. Commun. 49(2013) 4890-4892. |
| [26] | H.Y. Thu, W.Y. Yu, C.M. Che, Intermolecular amidation of unactivated sp2 and sp3 CH bonds via palladium-catalyzed cascade CH activation/nitrene insertion, J. Am. Chem. Soc. 128(2006) 9048-9049. |
| [27] | E.T. Nadres, O. Daugulis, Heterocycle synthesis via direct C-H/N-H coupling, J. Am. Chem. Soc. 134(2012) 7-10. |
| [28] | T. Kang, Y. Kim, D. Lee, et al., Iridium-catalyzed intermolecular amidation of sp3 C-H bonds:late-stage functionalization of an unactivated methyl group, J. Am. Chem. Soc. 136(2014) 4141-4144. |
| [29] | G. He, Y. Zhao, S. Zhang, et al., Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C (sp3)-H and C (sp2)-H bonds at g and d positions, J. Am. Chem. Soc. 134(2012) 3-6. |
| [30] | J.J. Neumann, S. Rakshit, T. Dröge, et al., Palladium-katalysierte amidierung nichtaktivierter C (sp3)-H-bindungen:von anilinen zu indolinen, Angew. Chem. Int. Ed. 121(2009) 7024-7027. |
| [31] | J. Pan, M. Suand, S.L. Buchwald, Palladium (0)-catalyzed intermolecular amination of unactivated C sp3-H bonds, Angew. Chem. Int. Ed. 50(2011) 8647-8651. |
| [32] | K.W. Fiori, J. Du Bois, Catalytic intermolecular amination of C-H bonds:method development and mechanistic insights, J. Am. Chem. Soc. 129(2007) 562-568. |
| [33] | C.G. Espino, J. Du Bois, A Rh-catalyzed C-H insertion reaction for the oxidative conversion of carbamates to oxazolidinones, Angew. Chem. Int. Ed. 113(2001) 618-620. |
| [34] | X. Ye, Z. He, T. Ahmed, et al., 1,2,3-Triazoles as versatile directing group for selective sp2 and sp3 C-H activation:cyclization vs substitution, Chem. Sci. 4(2013) 3712-3716. |
| [35] | Q. Zhang, K. Chen, W. Rao, et al., Stereoselective synthesis of chiral α-amino-β-lactams through palladium(II)-catalyzed sequential monoarylation/amidation of C (sp3)-H bonds, Angew. Chem. Int. Ed. 52(2013) 13588-13592. |
| [36] | G. He, S.Y. Zhang, W.A. Nack, et al., Use of a readily removable auxiliary group for the synthesis of pyrrolidones by the palladium-catalyzed intramolecular amination of unactivated γ C (sp3)-H bonds, Angew. Chem. Int. Ed. 52(2013) 11124-11128. |
| [37] | A. McNally, B. Haffemayer, B.S.L. Collins, et al., Palladium-catalysed CH activation of aliphatic amines to give strained nitrogen heterocycles, Nature 510(2014) 129-133. |
| [38] | M. Yang, B. Su, Y. Wang, et al., Silver-catalysed direct amination of unactivated C-H bonds of functionalized molecules, Nat. Commun. 5(2014) 4707-4712. |
| [39] | C.P. Allen, T. Benkovics, A.K. Turek, et al., Oxaziridine-mediated intramolecular amination of sp3-hybridizedC-Hbonds, J.Am. Chem. Soc.131(2009) 12560-12561. |
| [40] | Q. Michaudel, D. Thevenet, P.S. Baran, Intermolecular Ritter-type C-H amination of unactivated sp3 carbons, J. Am. Chem. Soc. 134(2012) 2547-2550. |
| [41] | Q. Li, Y. Huang, T. Chen, et al., Copper-catalyzed aerobic oxidative amination of sp3 C-H bonds:efficient synthesis of 2-hetarylquinazolin-4(3H)-ones, Org. Lett. 16(2014) 3672-3675. |
| [42] | B.L. Tran, B. Li, M. Driess, et al., Copper-catalyzed intermolecular amidation and imidation of unactivated alkanes, J. Am. Chem. Soc. 136(2014) 2555-2563. |
| [43] | X. Wu, Y. Zhao, G. Zhang, et al., Copper-catalyzed site-selective intramolecular amidation of unactivated C (sp3)-H bonds, Angew. Chem. Int. Ed. 53(2014) 3706-3710. |
| [44] | R.T. Gephart, D.L. Huang, M.J.B. Aguila, et al., Catalytic C-H amination with aromatic amines, Angew. Chem. Int. Ed. 51(2012) 6488-6492. |
| [45] | Z. Wang, J. Ni, Y. Kuninobu, et al., Copper-catalyzed intramolecular C. H and C (sp2)-H amidation by oxidative cyclization, Angew. Chem. Int. Ed. 53(2014) 3496-3499. |
| [46] | S.N. Gavade, R.S. Balaskar, M.S. Mane, et al., An efficient method for the N-arylation of phenylurea via copper catalyzed amidation, Chin. Chem. Lett. 22(2011) 675-678. |
| [47] | W. Liu, L.Y. Hai, R.L. Liu, et al., Copper-catalyzed N-arylation of 2-arylindoles with aryl halides, Chin. Chem. Lett. 25(2014) 1240-1243. |
| [48] | C. Huang, Y. Fu, H. Fu, et al., Highly efficient copper-catalyzed cascade synthesis of quinazoline and quinazolinone derivatives, Chem. Commun. 47(2008) 6333-6335. |
| [49] | X. Liu, H. Fu, Y. Jiang, Y. Zhao, A simple and efficient approach to quinazolinones under mild copper-catalyzed conditions, Angew. Chem. Int. Ed. 48(2009) 348-351. |
| [50] | X. Yang, H. Liu, H. Fu, et al., Efficient copper-catalyzed synthesis of 4-aminoquinazoline and 2,4-diaminoquinazoline derivatives, Synlett 1(2010) 101-106. |
| [51] | V.L. Truong, M. Morrow, Mild and efficient ligand-free copper-catalyzed condensation for the synthesis of quinazolines, Tetrahedron Lett. 51(2010) 758-760. |
| [52] | J. Ju, R. Hua, J. Su, Copper-catalyzed three-component one-pot synthesis of quinazolines, Tetrahedron 68(2012) 9364-9370. |
| [53] | L. Zhang, J.H. Su, S. Wang, et al., Direct electrochemical imidation of aliphatic aminesvia anodic oxidation, Chem. Commun. 47(2011) 5488-5490. |
| [54] | Z. Zhang, J. Su, Z. Zha, et al., Electrochemical synthesis of the aryl a-ketoesters from acetophenones mediated by KI, Chem. Eur. J. 19(2013) 17711-17714. |
 2015, Vol.26
2015, Vol.26 



