b Department of Medicinal Chemistry, Faculty of Pharmacy, El-Minia University, 61519 El-Minia, Egypt;
c Institute of Organic Chemistry, Karlsruhe Institute of Technology, Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany;
d Laboratory of Inorganic Chemistry, Department of Chemistry, University of Helsinki, 00014 Helsinki, Finland
Thiadiazole is a prevalent and important five-membered heterocyclic system. There are several isomers of thiadiazole. A glance at standard reference works shows that 1, 3, 4-thiadiazole has been investigated more than other isomers [1].
The most familiar thiadiazole containing compound could be acetazolamide, the famous carbonic anhydrase inhibitor currently used in treatment of glaucoma [2], high-altitude illness [3], cancer [4, 5], HIV [4], seizures [6], diabetes [7], and hypertension [8]. Synthesis of 1, 3, 4-thiadiazoles usually involves multi-step procedures such as cyclization of thiosemicarbazide with di-(2- pyridyl)thionocarbonate (DPT), dicyclohexylcarbodimide (DCC) [9]. Oxidative cyclization of thiosemicarbazides with FeCl3 [10] or reaction of thiosemicarbazides and CS2 under reflux [11] have been reported. Treatment of isothiocyanates with pure lithiated (trimethylsilyl)diazomethane (Me3SiCN2Li) in Et2O afforded 2- amino substituted-1, 3, 4-thiadiazoles [12, 13].
Although, there are many reports for the synthesis of 1, 3, 4- thiadiazoles [14, 15], few reports are available for the synthesis of 5-unsubstituted 1, 3, 4-thiadiazoles. Diethyl chlorophosphate in DMF was proposed as a cyclizing agent for the synthesis of 5- unsubstituted 1, 3, 4-thiadiazoles [16]. The latter were formed in moderate yields by cyclization of thiohydrazides. 5-Unsubstituted 1, 3, 4-thiadiazoles are of interest as biologically active compounds [17, 18]. Further, they are used in the synthesis of various heterocyclic compounds [19, 20].
2. Experimental 2.1. General experimental procedureMelting points were determined using open glass capillaries on a Gallenkamp melting point apparatus and are uncorrected. The IR spectra (KBr discs) were recorded on a Bruker FT-IR and Shimadzu 408 instruments. 1H NMR and 13C NMR spectra were obtained using a Bruker AM 400 spectrometer (400 MHz for 1H NMR and 100 MHz for 13C NMR) with tetramethylsilane as the internal standard; the chemical shifts are expressed in δ and coupling constants in Hz. The 13C NMR assignments (q = quaternary carbon atoms) were made with the aid of DEPT 135/90 spectra. Mass spectra (70 eV, electron impact mode) were recorded on a Finnigan MAT 312 instrument. Elemental analyses were carried out at the Microanalytical Center, Cairo University, Egypt. Preparative layer chromatography (plc) used air-dried 1.0 mm thick layer of slurry applied silica gel (Merck Pf254) on 48 cmwide and 20 cm high glass plates using the solvent listed.
2.2. Starting materials2, 3, 5, 6-Tetrachloro-1, 4-benzoquinone (CHL-p, 2) was purchased from Fluka. 2-(Hydrazinecarbonothioyl)-N-aryl/alkyl hydrazinecarbo-thioamides 1a-d were prepared according to published procedures for preparation compounds 1a [21] and 1b-d [22].
2.3. Reaction of 1a-d with 2, 3, 5, 6-tetrachloro-1, 4-benzoquinone (2)2-(Hydrazinecarbonothioyl)-N-substituted hydrazinecarbothioamides 1a-d (1 mmol) in 20 mL dry ethyl acetate was added to 246 mg (1 mmol) (CHL-p, 2) in 20 mL dry ethyl acetate at room temperature. The reaction mixture was stirred for 3 h. After standing for 24 h, the precipitate was filtered, washed with cold ethyl acetate and identified as 2, 3, 5, 6-tetrachloro-p-benzohydroquinone 4 [23]. The filtrate was pre-concentrated, then applied to 3 plc plates and developed using toluene/ethyl acetate (fr = 10:2 for 1a, 1b, and fr = 10:4 for 1c, 1d) to give N-substituted-1, 3, 4- thiadiazol-2-amines 3a-d, extracted by acetone and recrystallized from listed solvents.
N-Phenyl-1, 3, 4-thiadiazol-2-amine (3a):Red crystals (acetonitrile), yield 87% (0.154 g), mp: 173 ℃ (Iit. 171-172 ℃) [16, 20].
N-Benzyl-1, 3, 4-thiazol-2-amine (3b): Red crystals (acetonitrile), yield 89% (0.170 g), mp: 109-110 ℃ (lit. 108 ℃) [19]. IR (KBr, cm-1): ν 3421-3380 (NH str.), 1611 (C=N str.). 1H NMR (400 MHz, DMSO-d6): δ 8.35 (br, 1H, NH-CH2Ph), 8.20 (s, 1H, CH=N), 7.36- 7.31 (m, 3H, Ar-H), 7.29-7.28 (m, 2H, Ar-H), 4.51 (s, 2H, CH2). 13C NMR (100 MHz, DMSO-d6): d 168.5 (C=N), 142.3 (CH=N), 138.7 (Ar-C), 128.3, 127.5, 127.0 (Ar-CH), 48.3 (CH2-Ph). MS m/z (%) 191 (M+, 70), 163 (11), 86 (100), 91 (13), 76 (10).
N-Allyl-1, 3, 4-thiazol-2-amine (3c): Red crystals (acetonitrile), yield 85% (0.112 g), mp: 74-75 ℃ (lit. 73 ℃) [19]. IR (KBr, cm-1): ν 3426-3384 (NH str.), 1600 (C=N str.). 1H NMR (400 MHz, DMSOd6) d 8.10 (br, 1H, NH-allyl), 7.90 (s, 1H, CH=N), 5.90-5.89 (m, 1H, allyl-CH=), 5.15-5.11 (m, 2H, allyl-CH2=), 3.92-3.90 (m, 2H, allyl- CH2N). 13C NMR (100 MHz, DMSO-d6): δ 167.6 (C=N), 142.4 (CH=N), 135.00 (allyl-CH), 115.45 (allyl-CH2=), 46.05 (allyl- CH2N). MS m/z (%) 141 (M+, 30), 113(28), 86 (100), 55 (40), 41 (75). N-Ethyl-1, 3, 4-thiazol-2-amine (3d): Red crystals (acetonitrile), yield 84% (0.108 g), mp: 71-72 ℃ (lit. 70 ℃) [24]. IR (KBr, cm-1): ν 3424-3372 (NH str.), 1601 (C=N str.). 1H NMR (400 MHz, DMSOd6): d 8.20 (br, 1H, NH-C2H5), 7.96 (s, 1H, CH=N), 3.49 (q, 2H, CH2, J = 7.50 Hz), 1.19 (t, 3H, J = 7.50 Hz). 13C NMR (100 MHz, DMSOd6): δ 166.8 (C=N), 143.1 (CH=N), 40.1 (CH2), 14.3 (CH3). MS m/z (%) 129 (M+, 55), 101 (40), 86 (100), 43 (50), 29 (80).
2, 3, 5, 6-Tetrachlorobenzene-1, 4-diol (4) [23]: mp: 230-232 ℃ (lit. 232 ℃).
2.4. Single crystal X-ray structure determination of 3aSingle crystals were obtained by recrystallization from acetonitrile. The single crystal X-ray diffraction study was carried out on an Agilent SuperNova diffractometer with EOS detector at 173 K using Mo kα radiation (λ = 0.71073Å ). Direct methods (SHELXS- 98) [25] were used for structure solution and refinement was carried out using SHELXL-2013 [25] (full-matrix least-squares on F2). Hydrogen atoms were localized by different Fourier synthesis map and refined using a riding model [H(N) free]. A semi-empirical absorption correction was applied.
3a: C8H7N3S, M = 177.23 g mol-1, red crystal, size 0.40 mm × 0.06 mm × 0.06 mm, monoclinic space group P21/n (no. 14), a = 11.3811(5)Å , b = 5.3161(2)Å , c = 14.3650(6)Å , β = 112.249(5)Å , V = 798.82(6) Å3, Z = 4, Dcalcd = 1.474 mg m-3, F(000) = 368, μ = 0.344 mm-1, T = 173 K, 3093 measured reflection (2θmax = 58.88), 1978 independent [Rint = 0.015], 112 parameters, 1 restraint, R1 [for 1604 I > 2σ(1)] = 0.043, wR2 (for all data) = 0.097, S = 1.07, largest diff. peak and hole = 0.232 eÅ -3/-0.218 eÅ -3.
Crystallographic data (excluding structure factors) for the structure reported in this work has been deposited with Cambridge crystallographic Data Center on supplementary publication no. CCDC-1046364. Copies of the data can be obtained free of charge on application to the director, CCDC, 12 Union Road, Cambridge CB2 IEZ, UK (Fax: +44 1223 3360333; e-mail: deposit@ ccdc.cam.ac.uk).
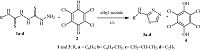
3. Results and discussionFor the synthesis of 5-unsubstituted 1, 3, 4-thiadiazoles as will be outlined in detail below, we report the heterocyclization of thiocarbonohydrazides 1a-d using 2, 3, 5, 6-tetrachloro-1, 4-benzoquinones (CHL-p, 2) as a reaction mediator.
After adding ethyl acetate solutions of 1a-d to an equimolar quantity of 2, 3, 5, 6-tetrachloro-1, 4-bezoquinone (CHL-p, 2), in ethyl acetate and letting it stand for 24 h at room temperature, the initial green colored turned to violet, which gradually changed to reddish brown. The isolated compounds 3a-d (Scheme 1) were characterized by their IR, 1H NMR, 13C NMR, mass spectral data and single X-ray crystallography.
|
Download:
|
| Scheme 1.Reaction of 1a–d with CHL-p (2). | |
The IR spectrum of 3c as an example was characterized by the presence of broad NH at 3426-3384, the band at 1600 was assigned to C=N stretching. The bands attributed to C=S stretching vibrations were not observed in the IR spectra of 3a-d.
The chemical shifts obtained from 1H NMR spectrum of 3c supported the proposed structure. Resonance assigned to allyl group was detected at 3.92-3.90 (allyl-CH2N), 5.15-5.11(allyl- CH2=) and 5.90-5.89 (allyl-CH=). The 1H NMR clearly showed singlet signal at 7.90 due to CH=N, whereas a broad band with D2O exchangeable observed at 8.10 for NH-allyl.
13C NMR data of representative compounds 3a-d which were obtained using DEPT technique at 100 MHz, also support the carbon framework by discrimination of CH2, CH and quaternary carbons. The 13C NMR of 3c showed downfield signals at 167.60, 142.40, 135.00 and 115.45 attributed to C=N, CH=N, allyl-CH= and allyl-CH2=respectively. The upfield signal resonated at 46.05 due to (allyl-CH2N).
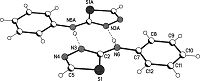
N-Phenyl-1, 3, 4-thiadiazol-2-amine (3a) was confirmed unambiguously by single crystal X-ray structure analysis (Fig. 1 and Tables S1-S7 in Supporting information) (note that the crystallographic numbering does not correspond to systematic IUPAC numbering rules).

|
Download:
|
| Fig. 1.Molecular structure of 3a in the crystal (one crystallographic independent molecule is shown; displacement parameters are drawn at 50% probability level). | |
The S(1)-C(5) bond length of 1.726(2)Å and S(1)-C(2) 1.7323(19) has single bond character, whereas bond lengths C(2)-N(3) 1.318(2)Å , C(5)-N(4) 1.285(3)Å suggest that these bonds have double bond character, as they comparable to C=N bond. The 2-phenyl amino-1, 3, 4-thiadiazole molecule (3a) is planar (mean deviation from the L.S. plane through all non hydrogen atoms 0.042Å , angle between the L.S: planes of the 1, 3, 4- thiadiazole ring and the phenyl ring 3.08). Intermolecular hydrogen bonding between N6A, N3A from one molecule to N3 and N6 of another forms a dimer with Ci-symmetry (Fig. 2).
|
Download:
|
| Fig. 2.Asymmetric unit of 3a with intermolecular hydrogen bonds shown as dotted lines. | |
Since the aforementioned reactions do not take place when no 2 is added to the solution of 1a-d in ethyl acetate, the presence of (CHL-p, 2), is definitely required for the transformation observed. Charge-transfer complexes may (but not necessarily have to) play an intermediate role. Since the cyclization involves intramolecular attacks on the thiocarbonyl group, it is conceivable that (CHL-p, 2) accelerates the process as a proton or a Lewis acid, possibly through intermediate 5 (Scheme 2), activating the respective C=S bond toward nucleophilic addition. This behavior may well be supported by the polar nature of the solvent stabilizing Zwitterionic adducts.

|
Download:
|
| Scheme 2.The mechanism of formation 5-unsubstituted-1, 3, 4-thiadiazoles. | |
An efficient and fast reaction between thiocarbonohydrazides 1a-d and CHL-p, 2 was observed to give 1, 3, 4-thiadiazoles. These oxidative cyclization reactions of 1a-d using CHL-p, which react as a mediator, provide the products in higher yields with lower costs. This environmentally benign procedure will be explored for other substrates.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.05.034.
| [1] | Y. Hu, C.Y. Li, X.M. Wang, Y.H. Yang, H.L. Zhu, 1,3,4-Thiadiazole:synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry, Chem. Rev. 114(2014) 5572-5610. |
| [2] | I.P. Kuar, R. Smitha, D. Aggarwal, M. Kapil, Acetazolamide:future perspective in topical glaucoma therapeutics, Int. J. Pharm. 248(2002) 1-14. |
| [3] | A.M. Luks, S.E. McIntosh, C.K. Grissom, et al., Wilderness Medical Society practice guidelines for the prevention and treatment of acute altitude illness:2014 update, Wilderness Environ. Med. 25(2014) S4. |
| [4] | D.E. Abdel Rahman, K.O. Mohamed, Synthesis of novel 1,3,4-thiadiazole analogues with expected anticancer activity, Der Pharma Chem. 6(2014) 323-335. |
| [5] | D. Kumar, G. Patel, A.K. Chavers, K.H. Chang, K. Shah, Synthesis of novel 1,2,4-oxadiazoles and analogues as potential anticancer agents, Eur. J. Med. 46(2011) 3085-3092. |
| [6] | H.N. Hafez, M.I. Hegad, I.S. Ahmed-Farag, A.B.A. El-Gazzar, A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-90,2-[1,3,4] thiadiazole derivatives as potential analgesic and anti-inflammatory agents, Bioorg. Med. Chem. Lett. 18(2008) 4538-4543. |
| [7] | P.A. Datar, T.A. Deokule, Design and synthesis of thiadiazole derivatives as antidiabetic agents, Med. Chem. 4(2014) 390-399. |
| [8] | M. Yusuf, R.A. Khan, B. Ahmed, Syntheses and anti-depressant activity of 5-amino-1,3,4-thiadiazole-2-thiol imines and thiobenzyl derivatives, Bioorg. Med. Chem. 16(2008) 8029-8034. |
| [9] | P. Puthiyapurayil, B. Poojary, C. Chikkanna, S.K. Buridipad, Design, synthesis and biological evaluation of a novel series of 1,3,4-oxadiazole bearing N-methyl-4-(trifluoromethyl)phenyl pyrazole moiety as cytotoxic agents, Eur. J. Med. Chem. 53(2012) 203-210. |
| [10] | X.M. Zhang, M. Qui, J. Sun, et al., Synthesis, biological evaluation, and molecular docking studies of 1,3,4-oxadiazole derivatives possessing 1,4-benzodioxan moiety as potential anticancer agents, Bioorg. Med. Chem. 19(2011) 6518-6524. |
| [11] | H. Rajak, R. Deshmukh, R. Veerasamy, et al., Novel semicarbazones based 2,5-disubstituted-1,3,4-oxadiazoles:one more step towards establishing four binding site pharmacophoric model hypothesis for anticonvulsant activity, Bioorg. Med. Chem. Lett. 20(2010) 4168-4172. |
| [12] | T. Aoyama, M. Kabeya, A. Fukushima, T. Shioiri, New method and reagents in organic synthesis. 53. Lithium trimethylsilyldiazomethane:a new synthon for the preparation of 2-amino-1,3,4-thiadiazoles from isothiocyanates, Heterocycles 23(1985) 2367-2369. |
| [13] | D.R. Armstrong, R.P. Davies, R. Haigh, et al., A solid-state, solution, and theoretical structural study of kinetic and thermodynamic lithiated derivatives of a simple diazomethane and their reactivities towards aryl isothiocyanates, Eur. J. Inorg. Chem. 2003(2003) 3363-3375. |
| [14] | C.Z. Zhao, J.L. Yu, X.L. Gu, H. Liu, Simultaneous determination of dihydroxybenzene isomers utilizing a thiadiazole filmelectrode, Chin. Chem. Lett. 25(2014) 370-374. |
| [15] | L.H. Shen, H.Y. Li, H.X. Shang, et al., Synthesis and cytotoxic evaluation of new colchicine derivatives bearing 1,3,4-thiadiazole moieties, Chin. Chem. Lett. 24(2013) 299-302. |
| [16] | V.N. Yarovenko, A.V. Shirokov, I.V. Zavarzin, et al., New cyclizing reagent for the synthesis of 1,3,4-thiadiazoles, Synthesis (2004) 17-19. |
| [17] | Y. Takatori, T. Yamaguchi, M. Nagakura, 2-b-Thenoylamido-1,3,4-thiadiazole, Jpn KoKai Tohkyo Koko 8028,946(1980), Chem. Abstr. 93(1980) 114536e. |
| [18] | R.C. Gadwood, M.R. Barbachyn, D.S. Toops, H.W. Smith, V.A. Vaillancourt, Preparation of azolylpiperaziylphenyloxazolidinones as antimicrobials, US pat. 5,736,545(1998), Chem. Abstr. 128(1998) 270612y. |
| [19] | D.H. Reid, F. Kurzer, Thiadiazoles and selenadiazoles, in:D.H. Reid (Ed.), Organic Compound of Sulphur, Selenium and Tellurium, Specialist Periodical Reports, vol. 3, The Chemical Society, London, 1975, pp. 670-707. |
| [20] | C.W. Bird, G.W.H. Cheeseman, P.A. Lowe, Condensation, in:C.W. Bird, G.W.H. Cheeseman (Eds.), Aromatic and Heteroaromatic, Specialist Periodical Reports, vol. 2, The Chemical Society, London, 1974, pp. 106-134. |
| [21] | M.K. Burghata, S.V. Gandhe, M.G. Ajmire, B.N. Berad, Synthesis of 20-(substituted) benzylidene hydrazine-5-arylamino-1,3,4-thiadiazoles and their antimicrobial activity, J. Indian Chem. Soc. 84(2007) 103-108. |
| [22] | A.A. Hassan, F.F. Abdel-Latif, M. Abdel-Aziz, et al., Efficient and facile synthesis of substituted aminothiadiazolylhydr-azonoindolin-2-ones, J. Heterocycl. Chem. (2015) (in press). |
| [23] | R. Pummerer, G. Schmidutz, H. Seifert, Institute of energy problems of chemical physics, RAS, Chem. Ber. 85(1952) 535-555. |
| [24] | R.A. Coburn, B. Bhooshan, R.A. Glennon, The preparation of 2-alkylamino-1,3,4-thiadiazoles, J. Org. Chem. 38(1973) 3947-3949. |
| [25] | (a) G.M. Sheldrick, A story history of Shelx, Acta Cryst. A 64(2008) 112-122;(b) G.M. Sheldrick, Crystal structure refinement with Shelx, Acta Cryst. C 71(2015) 3-8. |
 2015, Vol.26
2015, Vol.26 


