b School of Life Science, Xiamen University, Xiamen 361005, China
DNA logic gates, the basis of logic operations and the core components in molecular computers, hold great promise for gene regulation, biomarker detection and molecular diagnostics that have become more and more important to human cancers, genetic disease, and infectious diseases [1, 2, 3, 4, 5, 6]. Various DNA logic gates have been developed [7, 8, 9, 10, 11, 12]. Wanget al. designed a series of DNA logic gates based on toehold-mediated strand displacement and Gquadruplex that can autonomously control the coalescence and release of PPIX in vitro, an important molecule in photodynamic diagnosis and therapy [8]. Songet al. constructed logic gates based on two DNA origami nanostructures to perform microRNA analysis [3]. Willneret al. designed a series of logic gates using DNA scaffold, which are activated by either adenosine monophosphate or cocaine [9]. Xuet al. constructed a circular DNA logic gates through ligase-assisted strand displacement to detect the position of gold nanoparticles [10]. Until now, there has been no consensus for the best logic gate design method in terms of simplicity and robustness. There is a continuous need for new logic gate design to meet specific needs and situations.
In this work, we designed a novel linear DNA logic gates for microRNA diagnostics based on the combination of toeholdmediated strand displacement [13, 14, 15] and fluorescence resonance energy transfer (FRET). MicroRNAs (miRNAs), a class of 19- 24-nt-long non-coding RNA molecules, are important indicators of numerous activities, including developmental processes, host- pathogen interactions and disease pathogenesis [16, 17, 18]. As demonstration, we constructed logic gates through using two indictors of heart failure, microRNA-195 [19] and microRNA-21 [20] as the logic input and the fluorescence from the separation of fluorophore and quencher as logic output symbol. Two logic gates (YES and AND gate) were built in the basis of the mechanism.
2. Experimental2.1. Materials
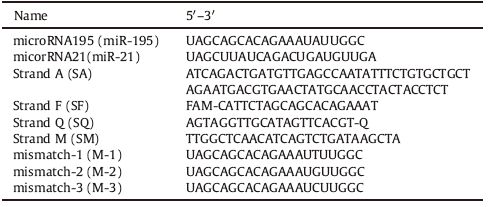
All synthesized and HPLC-purified sequences of oligos as depicted in Table 1 were commercially ordered from TaKaRa Bio Inc. (Dalian, China). MiR-195 [19] and miR-21 are two indictors of heart failure; SF and SQ are complementary to SA. M-1, M-2, and M-3 are three miR-195 analogs containing a single base different from the miR-195. All other reagents were of analytical grade. Deionized water was obtained from the Nanopure InfinityTM ultrapure water system (Barnstead/Thermolyne Corp., Dubuque, IA, USA). 0.1% DEPC water was prepared for RNA experiments.
|
|
Table 1 Sequence of oligos in this study. |
The base work unit of logic gate consists of 200 nmol/L SA (Strand A), 200 nmol/L SF (Strand F), and 200 nmol/L SQ (Strand Q) in a reaction buffer (20 mmol/L Tris-HCl pH 7.4, and 15 mmol/L MgCl2). The addition of 200 nmol/L SM in the base unit for AND gate. Input strands (200 nmol/L) were added into the base unit solution and incubated for 1 h to perform toehold-mediated strand displacement at room temperature.
2.3. Fluorescence measurementThe fluorescence intensities were recorded using F-7000 fluorescence spectrophotometer (Hitachi, Japan). The fluorescent spectra were measured using the spectrofluorophotometer. The excitation wavelength was 490 nm, and the spectra were recorded between 500 and 600 nm. The fluorescence emission intensity was measured at 520 nm.
3. Results and discussionOur proposed system is designed to determine whether only miR-195 or both miR-195 and miR-21 are present in a sample (Figs. 1-3). The miR-195 and miR-21 are designed as inputs of logic gates, and ‘‘+’’ or ‘‘-’’ as the logic outputs, indicating positive diagnostics or negative diagnostics, respectively. The base unit of our logic gate consists of three hybridization strands (Fig. 1). SA was a 69-mer oligonucleotide, containing partial recognition regions that were complementary with input strands miR-195 and miR-21. SF was marked with FAM fluorophore at the 50 end, and SQ was marked with Dabcyl quencher at the 30 end. When SF and SQ were in close proximity to each other through hybridization with SA, the fluorescence of the FAM was quenched by Dabcyl through FRET. After separation of SF and SQ, the fluorescence intensity increased accordingly.

|
Download:
|
| Fig. 1.Mechanisms of linear logic gate YES operation. (A) The base unit state is on the left of the arrows and the report states are shown on the right. FAM-labeled SF and Dcyble-labeled SQ are complementary to SA. (B) Fluorescence emission spectra of different states. (C) The fluorescence intensities are listed in a truth table. (1) SF + SQ + SA;(2) SF + SQ + SA + miR-195. | |

|
Download:
|
| Fig. 2.Mechanisms of linear logic gate AND operation. (A) The base state is on the left of the arrows and the report states are shown on the right. (B) Fluorescence emission spectra of different states. (C) The fluorescence intensities are listed in a truth table. (1) SF + SQ + SA + SM; (2) SF + SQ + SA + SM + miR-21; (3) SF + SQ + SA + SM + miR-195;(4) SF + SQ + SA + SM + miR-21 + miR-195. The error bars showed the standard deviation of three replicate determinations. | |

|
Download:
|
| Fig. 3.The specificity of the logic gate. (A) Fluorescence emission spectra. (B) The fluorescence intensities are listed in a truth table. (1) SF + SQ + SA; (2) SF + SQ + SA + M-1; (3)SF + SQ + SA + M-2; (4) SF + SQ + SA + M-3; (5) SF + SQ + SA + miR-195. The error bars showed the standard deviation of three replicate determinations. | |
We first tried the YES gate (Fig. 1), a simple logic device. The YES gate can be interpreted as ‘‘turn-on’’ model of single analyst diagnosis [8]. The output signal is obtained when the single input is presented (Fig. 1A). Here, the fluorescence of the FAM of SF was quenched when SF and SQ were hybridized with SA, which indicated the YES gate was ready for miR-195 detection. After input of miR-195, it would displace the SF by toehold-mediated strand displacement, which caused the separation of SF/SQ and the fluorescence intensity increased significantly (Fig. 1B). This proved that the system could work as a YES gate with an input of miR-195 and output of the fluorescent signal (Fig. 1C). Similarly, the detection miR-21 can be achieved by designing such a YES gate.
Then we tested the AND gate using this system (Fig. 2). AND logic is represented by the situation where the output of the gate occurs only when both inputs are present. The AND logic gate queries two microRNAs to determine whether both of them are present [10]. The AND gate in our system consists of SA, SF, SQ and SM. Experiments were performed by testing the AND gate with all possible input combinations of miR-195 and miR-21. Fig. 2 shows the different output results of the AND gate. The addition of miR-21 displaced only SM, leaving SF and SQ anchored to the SA (Fig. 2A). Since there was no separation between fluorophore and quencher, no fluorescence signal was produced. Moreover, if input miR-195 alone was added, the gate state did not change, as evidenced by no significant fluorescence signal, because there was no exposed toehold region. Therefore, adding either miR-21 or miR-195 input strands did not result in an increase of fluorescence intensity. However, when both input miR-21 and miR-195 were added at the same time, miR-21 occupied SM by strand displacement. Then, the toehold regions for miR-195 on SA were exposed. Subsequently, the miR-195 displaced SF and caused the separation of quencher and fluorophore and increased significantly the fluorescence intensity (Fig. 2B). The fluorescence signal produced suggested that such multilevel displacements operated effectively in the AND logic gate (Fig. 2C).
The specificity of the system is very important for our clinic diagnostics in near future. We used three miR-195 analogs containing mismatched nucleotides to investigate the specificity of logic gate (Fig. 3). Three miR-195 analogs (M-1, M-2, and M-3) are single base difference from the miR-195, respectively (Table 1). The allele single-base mismatched sequences were designed such that the mismatched site located in the seventh base of toehold region from 50-end. It was shown in Fig. 3 that the fluorescence signal was significantly larger in the solution containing the miR- 195 than that of other analogs containing mutant base, indicating the high specificity of the logic gates.
4. ConclusionIn summary, we have constructed a logic gate system based on FRET and toehold-mediated strand displacement, and demonstrated that the logic results are easily detected by fluorescence signals for microRNA diagnostics. The logic gates can perform simple computing and display correct symbols in response to disease indicators. Adopting the toehold-mediated strand displacement, the proposed logic gate performed perfectly the output signal without enzyme. Moreover, in principle, any microRNA, even any DNA sequence including specific gene fractions for some diseases, can be used as the input for our built logic system for detection and diagnostics. This indicates that our logic gates have the potential for biological sample application.
AcknowledgmentsThis work is supported by National Natural Science Foundation of China (No. 21275043) and National Basic Research Program of China under Grants (No. 2009CB421601).
| [1] | Y. Benenson, T. Paz-Elizur, R. Adar, et al., Programmable and autonomous computing machine made of biomolecules, Nature 414(2001) 430-434. |
| [2] | M. Privman, T.K. Tam, M. Pita, E. Katz, Switchable electrode controlled by enzyme logic network system:approaching physiologically regulated bioelectronics, J. Am. Chem. Soc. 131(2009) 1314-1321. |
| [3] | D.F. Wang, Y.M. Fu, J. Yan, et al., Molecular logic gates on DNA origami nanostructures for MicroRNA diagnostics, Anal. Chem. 86(2014) 1932-1936. |
| [4] | B. Gil, M. Kahan-Hanum, N. Skirtenko, R. Adar, E. Shapiro, Detection of multiple disease indicators by an autonomous biomolecular computer, Nano Lett. 11(2011) 2989-2996. |
| [5] | T. Omabegho, R.J. Sha, N.C. Seeman, A bipedal DNA Brownian motor with coordinated legs, Science 324(2009) 67-71. |
| [6] | L.X. Mu, W.X. Shi, G.W. She, J.C. Chang, S.T. Lee, Fluorescent logic gates chemically attached to silicon nanowires, Angew. Chem. Int. Ed. 48(2009) 1-4. |
| [7] | J. Yang, L.J. Shen, J.J. Ma, et al., Fluorescent nanoparticle beacon for logic gate operation regulated by strand displacement, ACS Appl. Mater. 5(2013) 5392-5396. |
| [8] | J.B. Zhu, L.B. Zhang, T. Li, S.J. Dong, E.K. Wang, Enzyme-free unlabeled DNA logic circuits based on toehold-mediated strand displacement and split G-quadruplex enhanced fluorescence, Adv. Mater. 25(2013) 2440-2444. |
| [9] | X.Q. Liu, R. Aizen, R. Freeman, O. Yehezkeli, I. Willner, Multiplexed aptasensors and amplified DNA sensors using functionalized graphene oxide:application for logic gate operations, ACS Nano 6(2012) 3553-3563. |
| [10] | C. Zhang, J. Yang, J. Xu, Circular DNA logic gates with strand displacement, Langmuir 26(2010) 1416-1419. |
| [11] | M. Moshe, J. Elbaz, I. Willner, Sensing of UO22+ and design of logic gates by the application of supramolecular constructs of iondependent DNA zymes, Nano Lett. 9(2009) 1196-1202. |
| [12] | H. Pei, L. Liang, G.B. Yao, et al., Reconfigurable three-dimensional DNA nanostructures for the construction of intracellular logic sensors, Angew. Chem. 124(2012) 9154-9158. |
| [13] | Z.F. Gao, Y. Ling, L. Lu, et al., Detection of single-nucleotide polymorphisms using an ON-OFF switching of regenerated biosensor based on a locked nucleic acidintegrated and toehold-mediated strand displacement reaction, Anal. Chem. 86(2014) 2543-2548. |
| [14] | J. Zhu, Y.S. Ding, X.T. Liu, L. Wang, W. Jiang, Toehold-mediated strand displacement reaction triggered isothermal DNA amplification for highly sensitive and selective fluorescent detection of single-base mutation, Biosens. Bioelectr. 59(2014) 276-281. |
| [15] | Y.S. Jiang, S. Bhadra, B.L. Li, A.D. Ellington, Mismatches improve the performance of strand-displacement nucleic acid circuits, Angew. Chem. Int. Ed. 53(2014) 1845-1848. |
| [16] | V. Ambros, The functions of animal microRNAs, Nature 431(2004) 350-355. |
| [17] | R.C. Friedman, K.K.H. Farh, C.B. Burge, D.P. Bartel, Most mammalian mRNAs are conserved targets of microRNAs, Genome Res. 19(2009) 92-105. |
| [18] | R. Schickel, B. Boyerinas, S.M. Park, M.E. Peter, MicroRNAs:key players in the immune system, differentiation, tumorigenesis and cell death, Oncogene 27(2008) 5959-5974. |
| [19] | E. Van Rooij, L.B. Sutherland, N. Liu, et al., A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure, Proc. Natl. Acad. Sci. U. S. A. 103(2006) 18255-18260. |
| [20] | T. Thum, P. Galuppo, C. Wolf, et al., MicroRNAs in the human heart, Circulation 116(2007) 258-267. |
 2015, Vol.26
2015, Vol.26