b Key Laboratory of Natural Medicine and Immuno-Engineering of Henan Province, Institute of Fine Chemical and Engineering, Henan University, Kaifeng 475004, China
Graphene has attracted dramatic attention in the past decade owing to its exceptional physical properties, such as significant electronic, mechanical and thermal properties, which offer various applications in many fields [1,2,3]. Particularly, graphene-based films have demonstrated intriguing potentials in nanocomposites, transparent conducting films, field effect transistors, batteries and supercapacitors [4,5]. In general, the fabrication of the films could be realized by spin-coating, dip-coating, vacuum filtration and layer-by-layer (LBL) assembly. LBL technique, a popular method in preparing muitilayers [6], has been widely used in constructing composite films involved graphene owing to the advantages of controllable thickness and components [7]. For the graphenebased muitilayers from LBL assembly, the driving force usually originates from electrostatic attraction between the graphene derivatives, such as graphene oxide (GO), and polymers bearing cationic groups [8,9,10,11,12]. In addition, LBL technique has also been transferred to spherical template to prepare graphene-based core- shell structure and the subsequent removal of the template could yield hollow graphene capsules [13], just like the preparation of carbon nanotube composite capsules [14,15,16]. However, the multilayer film from the LBL assembly are not very stable due to the relatively weak electrostatic interaction between the layers, the post-treatment for covalent linking could be considered to significantly improve the stability of the films [17].
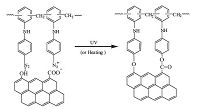
Diazoresin (DR) with light- and heat-sensitive diazonium group has been utilized in fabricating stable multilayer films through LBL assembly wherein the hydrogen bond or ionic bond between DR and the opponent could convert to covalent bond under UV irradiation or heating [18,19,20]. In this letter, we repot the LBL deposition of GO and DR on planar substrates and colloid spherical templates. Upon UV irradiation or heating, the ionic bonds and hydrogen bonds between GO and DR transform into covalent bonds (Fig. 1), leading to the cross-linking of the GO/DR composite film and shell. The subsequent solvent dissolution of the colloidal cores would yield hollow GO microspheres.
|
Download:
|
| Fig. 1.Schematic representation of the bonds transformation between GO and DR upon UV irradiation or heating. | |
Poly(allylamine hydrochloride (PAH, MW: 120, 000-200, 000) and poly(sodium 4-styrenesulfonate) (PSS, MW~70, 000) were purchased from Sigma-Aldrich and used as received. The monodisperse polystyrene (PS) microsphere templates were prepared by a dispersion polymerization method [14] and DR was synthesized by the means of literature [18]. GO was prepared through a modified Hummer’s method [21].
The quartz slides and silicon wafers to be used for planar LBL assembly were first heated in a freshly prepared piranha solution (a 1:3 mixture of 30% H2O2 and 98% H2SO4) and then thoroughly washed with deionized water. The LBL procedure was carried out by immersing the substrates in the aqueous solution of DR (2.0 mg/ mL) for 5 min and rinsing with deionized water before immersing the substrates in an aqueous dispersion of GO (1.0 mg/mL) for 5 min and water rinsing. By repeating the assembly cycle, multilayered DR/GO thin films were fabricated on the substrates.
The assembly of DR/GO on the PS colloid templates was realized in following process. 10 mg of PS microspheres in 20 mL of aqueous PAHsolution(2.0mg/mL)was slowly stirred for 2 h before removing excess PAH by repeated centrifugation and water rinsing. Thereafter, the PAH-modified PS microspheres were covered by a PSS layer in 20 mLof aqueous PSS solution (2.0mg/mL) by the samefashion. The negatively charged microsphereswere then slightly stirred in 20 mL of aqueous DR solution (1.0mg/mL) for 30 min. After collected by centrifugation and rinsing, the sample was immersed in 20mL of aqueous GO solution (2.0mg/mL) for 30 min, followed by a centrifugation and rinsing process. By the alternate assembly of DR and GO respectively, the DR/GO multilayers were deposited on the PS microspheres to fabricate the core-shell structure. To avoid the decomposition of light-sensitive DR, the LBL processes involved DR were carried out in a darkroom.
To convert the ionic bonds and hydrogen bonds between GO and DR to covalent bonds, UV irradiation for 20 min or heating at 120 8C for 2 h could be used. In present work, UV irradiation was preferred because of the easy operation. To remove the PS cores, the microspheres covered with DR/GO multilayers were stirred in toluene for 5 h.
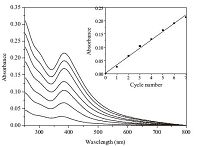
3. Results and discussionThe hydroxyl and carboxyl groups of GO could interact with the diazonium groups of DR through hydrogen and ionic bonds, respectively, enabling the effective LBL assembly of DR and GO. The assembly procedures on a quartz slide could be investigated by UV-vis spectrometry, as shown in Fig. 2. It is obvious that the absorption of the film increases stepwise with the increase of the LBL cycle. The feature absorbance at 380 nm is attributed to the p- p* transition of the strong conjugation between the diazonium group and the phenyl group of DR. As the number of bilayer on quartz slide increases, the absorbance at 380 nm increases linearly (inset in Fig. 2), indicating the uniform DR deposition in each cycle.
|
Download:
|
| Fig. 2.UV–vis absorption spectra of DR/GO multilayer films fabricated on a quartz slide. The number of cycle is 1–7 from bottom to top. The inset shows the absorbance at 380 nm versus the cycle number. | |
Due to the sensitivity of the diazonium group toward light and heat, the ionic bonds or hydrogen bonds between DR and GO would gradually transform into covalent bonds under UV irradiation or heating (Fig. 1). Fig. 3 exhibits the evolution of absorption spectrum of the (DR/GO)6 films upon UV irradiation. Obviously, the absorption at 380 nm decreases during the irradiation duration, indicating the decomposition of diazonium groups. The inset of Fig. 3 reveals the relationship between ln[(A0-Ae/At-Ae)] and irradiation time t, where A0, At and Ae represents the absorption at the beginning of, the time t of, and the end of the irradiation, respectively. The linear fitting implies that the photo decomposition reaction is in good accordance with the first-order kinetics.

|
Download:
|
| Fig. 3.UV–vis absorption spectra of a six-bilayered DR/GO film for different UV irradiation time of (from top to bottom) 0, 0.5, 1, 1.5, 2, 2.5, 3 and 4 min. Inset is the plot of ln[(A0-Ae)/(At-Ae)] against irradiation time. | |
The transformation from weak non covalent bonds to strong covalent bonds could dramatically enhance the stability of the DR/ GO composite film. The film on a quartz slide without irradiation or heating would be completely etched after sonicated in DMF for only a few seconds. While for the cross-linked film, it could be well kept in DMF under sonication for 20 min.
The LBL assembly of DR/GO is also realized on spherical PS templates. The stepwise growth of DR/GO multilayer on PS could be monitored through SEM. As shown in Fig. 4a, after a DR/GO assembly cycle, the smooth surface of the PS turns a little rough due to the deposition of GO. When five DR/GO assembly cycle is finished, the microsphere is covered by wrinkled DR/GO multilayer, suggesting the successful LBL assembly on the spherical template and the formation of core-shell structure (Fig. 4b). UV irradiation or heating could also be used to cross-link the shell of the composite microsphere and hollow DR/GO microspheres or microcapsules are expected by removing the PS template via dissolution in organic solvents. Fig. 4c shows the SEM image of the DR/GO composite after dissolving the PS template cores without UV irradiation or heating. Obviously, owing to the lost of the support of PS, almost all of the flexible DR/GO shell collapse and lose the spherical structure, presenting the morphologies of microcapsules or semi-microspheres. While for the DR/GO composite after UV irradiation, the dissolution of the PS cores yields lots of hollow microspheres (Fig. 4d), which inherit the spherical shell structure of their precursors. The existence of the perfect hollow microspheres (inset in Fig. 4d) should be ascribed to the formation of covalent cross-linking in the DR/GO shell.
|
Download:
|
| Fig. 4.SEM images of PS microspheres after one (a) and five (b) DR/GO assembly cycle(s), and SEM images the DR/GO multilayer composites from toluene dissolution of (DR/ GO)5PS microspheres before (c) and after (d) UV-irradiation. Inset of (d) shows the image in high magnification. | |
In summary, we have demonstrated that LBL assembly of DR and GO through ionic bond and hydrogen bond interaction could be carried out on 2-D substrate and 3-D microspheres. Covalent cross-linking of DR and GO by UV irradiation or heating would effectively strengthen the multilayer composites. In particular, the dissolution removal of PS templates of core-shell microsphere structure with cross-linked DR/GO shell results in hollow DR/GO microspheres and semi-microspheres. Our results might be useful for the researches of graphene-related multilayer materials.
AcknowledgmentsThis work was supported by the Natural Science Foundation of China (Nos. 21173266 and 21473250) and the Fundamental Research Funds for the Central Universities, the Research Funds of Renmin University of China (No. 11XNJ021).
| [1] | A.K. Geim, Graphene:status and prospects, Science 324(2009) 1530-1534. |
| [2] | C.N.R. Rao, A.K. Sood, K.S. Subrahmanyam, A. Govindaraj, Graphene:the new twodimensional nanomaterial, Angew. Chem. Int. Ed. 48(2009) 7752-7777. |
| [3] | M.J. Allen, V.C. Tung, R.B. Kaner, Honeycomb carbon:a review of graphene, Chem. Rev. 110(2010) 132-145. |
| [4] | D.Q. Wu, F. Zhang, H.W. Liang, X.L. Feng, Nanocomposites and macroscopic materials:assembly of chemically modified graphene sheets, Chem. Soc. Rev. 41(2012) 6160-6177. |
| [5] | M. Yang, Y. Hou, N.A. Kotov, Graphene-based multilayers:critical evaluation of materials assembly techniques, Nano Today 7(2012) 430-447. |
| [6] | X. Zhang, H. Chen, H.Y. Zhang, Layer-by-layer assembly:from conventional to unconventional methods, Chem. Commun. (2007) 1395-1405. |
| [7] | J. Hong, J.Y. Han, H. Yoon, et al., Carbon-based layer-by-layer nanostructures:from films to hollow capsules, Nanoscale 3(2011) 4515-4531. |
| [8] | D. Li, M.B. Muller, S. Gilje, R.B. Kaner, G.G. Wallace, Processable aqueous dispersions of graphene nanosheets, Nat. Nanotech. 3(2008) 101-105. |
| [9] | T. Cassagneau, F. Gué rin, J.H. Fendler, Preparation and characterization of ultrathin films layer-by-layer self-assembled from graphite oxide nanoplatelets and polymers, Langmuir 16(2000) 7318-7324. |
| [10] | H.B. Yao, L.H. Wu, C.H. Cui, H.Y. Fang, S.H. Yu, Direct fabrication of photoconductive patterns on LBL assembled graphene oxide/PDDA/titania hybrid films by photothermal and photocatalytic reduction, J. Mater. Chem. 20(2010) 5190-5195. |
| [11] | J.F. Shen, Y.Z. Hu, C. Li, et al., Layer-by-layer self-assembly of graphene nanoplatelets, Langmuir 25(2009) 6122-6128. |
| [12] | Z.Y. Xiong, T.H. Gu, X.G. Wang, Self-assembled multilayer films of sulfonated graphene and polystyrene-based diazonium salt as photo-cross-linkable supercapacitor electrodes, Langmuir 30(2014) 522-532. |
| [13] | J. Hong, K. Char, B.S. Kim, Hollow capsules of reduced graphene oxide nanosheets assembled on a sacrificial colloidal particle, J. Phys. Chem. Lett. 1(2010) 3442-3445. |
| [14] | M.X. Tang, Y.J. Qin, Y.Y. Wang, Z.X. Guo, Hollow carbon nanotube microspheres and hemimicrospheres, J. Phys. Chem. C 113(2009) 1666-1671. |
| [15] | J.W. Cui, Y.Q. Liu, J.C. Hao, Multiwalled carbon-nanotube-embedded microcapsules and their electrochemical behavior, J. Phys. Chem. C 113(2009) 3967-3972. |
| [16] | A.L. Xiong, X. Lu, Y.M. Ma, et al., Cross-linked multilayer composite films and microcapsules embedded carbon nanotubes, Mater. Lett. 105(2013) 132-135. |
| [17] | A.A. Mamedov, N.A. Kotov, M. Prato, et al., Molecular design of strong single-wall carbon nanotube/polyelectrolyte multilayer composites, Nat. Mater. 1(2002) 190-194. |
| [18] | H. Luo, J.Y. Chen, G.B. Luo, Y.N. Chen, W.X. Cao, Self-assembly films from diazoresin and carboxy-containing polyelectrolytes, J. Mater. Chem. 11(2001) 419-422. |
| [19] | S.H. Qin, D.Q. Qin, W.T. Ford, J.E. Herrera, D.E. Resasco, Grafting of poly(4-vinylpyridine) to single-walled carbon nanotubes and assembly of multilayer films, Macromolecules 37(2004) 9963-9967. |
| [20] | J.H. Shi, Y.J. Qin, H.X. Luo, et al., Covalently attached multilayer self-assemblies of single-walled carbon nanotubols and diazoresins, Nanotechnology 18(2007) 365704. |
| [21] | B. Wang, X.L. Li, T.F. Qiu, et al., High volumetric capacity silicon-based lithium battery anodes by nanoscale system engineering, Nano Lett. 13(2013) 5578-5584. |
 2015, Vol.26
2015, Vol.26 


