Tetrahydrofuran (THF) is an important chemical intermediate. It is widely used both as a precursor to manufacture pyrrolidone, polyurethanes (PU), polytetramethylene ether glycol (PTMEG) and as a universal solvent in many fields. With the fast growing market for PTMEG, the demand for THF is further enhanced as a polymer monomer [1]. At present, THF is produced through the dehydration of 1, 4-butanediol (BDO) catalyzed by inorganic acid in industry [2]. The raw material BDO is mainly synthesized from Reppe process which needs stringent reaction conditions and high energy consumption [3]. Gas phase hydrogenation of dimethyl maleate (DMM) is a promising method to produce THF under much milder reaction conditions than those of Reppe process [1, 4]. Moreover, it has more advantages over the economic costs in consideration of the raw materials price. It is reported that maleic anhydride as the upstream product of dimethyl maleate can be obtained from direct oxidation of n-bu tane [5].
The whole reaction scheme of hydrogenation of DMM is shown in Scheme 1. The first hydrogenation of DMM occurs in C=C, producing dimethyl succinate (DMS). Subsequent hydrogenation of C=O of DMS yields to γ-butyrolactone (GBL), 1, 4-butanediol (BDO) and THF. Dehydration of BDO to THF needs a weak acidic site such as SiO2. Recently, the studies on the Cu/SiO2 catalysts have gradually attracted more and more attentions in hydrogenation of ester due to the excellent catalytic performance and low-cost copper resource. The reported researches of Cu/SiO2 catalysts are mainly about the preparation methods [6, 7, 8], the addition of promoters [9, 10, 11], the modification of supports [12, 13] and the copper valence state [14, 15, 16], aiming at obtaining a high dispersed Cu/SiO2 catalysts and finding out the active site in the hydrogenation of dimethyl oxalate (DMO). However, the systematic study about the copper valence state of the Cu/SiO2 catalyst has not yet been reported in hydrogenation of DMM.

|
Download:
|
| Scheme 1.The whole reaction scheme of hydrogenation of DMM. | |
In this paper, a series of Cu/SiO2 catalysts were prepared by different methods for the purpose of forming different copper valence states. The catalytic activity was valued via gas phase hydrogenation of DMM in a continuous flow unit with a fix-bed reactor. The surfaces copper valence state of Cu/SiO2 was discussed in detail and correlated with the turnover frequency (TOF) and characterization data.
2. Experimental 2.1. Catalyst preparationCu/SiO2 catalyst prepared by the wetness impregnation (WI) is as follows: 3.78 g Cu(NO3)2·3H2O was dissolved in 20 mL distilled water and 4 g silica was then added into copper aqueous solution. After impregnated for 12 h, the precursor was dried at 393 K for 8 h, and subsequently calcined at 723 K for 4 h. The obtained catalyst is denoted as WI-Cu/SiO2.
Cu/SiO2 catalyst prepared by the ammonia-evaporation (AE) [6] is as follows: 3.78 g Cu(NO3)2·3H2O was dissolved in 40 mL distilled water and 11.5 mL concentrated ammonia (28 wt.%) aqueous solution was then added to the copper aqueous solution under stirring for 30 min. And subsequently 4 g silica was added under continuous stirring. After stirred for 4 h at room temperature, the mixture was rapidly heated to 363 K for evaporation of the ammonia until the pH value decreased to 6.5. The precipitate was filtered, washed, dried at 393 K for 8 h, and calcined at 723 K for 4 h. The obtained catalyst is denoted as AE-Cu/SiO2.
2.2. Catalyst characterizationThe surface area and porosity analysis of the catalysts were measured with N2 physisorption at 77 K on a Micromeritics ASAP 2020. The exposed metallic Cu surface area was determined by N2O titration [17] using a thermal conductivity detector (TCD). The calculation of metallic Cu surface areas was based on the assumption that the shape of the copper particles was spherical and there were 1.46 × 1019 copper atoms per square meter. The Powder X-ray diffraction (XRD) patterns were collected on a PANalytical X’Pert diffractometer equipped with a Cu Ka radiation (λ = 0.1541 nm) with a scanning range of 108-808. The operating voltage was 40 kV, and the current was 40 mA. Fourier transform infrared (FT-IR) spectra of the calcined catalysts and CO species adsorbed on the activated catalysts were recorded on a Nicolet 6700 spectrometer. Before the adsorption of CO, the calcined catalysts were activated at 573 K in H2 atmosphere. The spectral resolution was 4 cm-1 and the scan time was 64. The surface copper species were analyzed by X-ray photoelectron spectroscopy (XPS) using a Kratos AXIS Ultra DLD spectrometer. The spectrum was detected with Al Ka line as the radiation source. The binding energy was referenced to the C1s peak at 284.6 eV.
2.3. Activity test and product analysisThe catalytic activity tests were valued in a fix-bed continuous flow reactor with a stainless steel tube (i.d. = 10 mm). 2 mL of catalyst (20-40 meshes) was loaded in the middle of the fix-bed, both sides packed with silica sand to ensure the flow welldistributed. The calcined catalyst was activated in pure hydrogen gas flow at 573 K for 4 h. After the temperature cold down to the reaction temperature of 513 K, 15 vol.% DMM/CH3OH was transported to a vaporizer by a high-pressure pump, mixed with hydrogen and flowed into the reaction tube at a system pressure of 5 MPa. The molar ratio of H2/DMM was 60, and the liquid hourly space velocity (LHSV) was 0.9 h-1. At last, the products were analyzed by Agilent GC 7890A fitted with a 30 m DB-1 capillary column equipped a flame ionization detector (FID).
3. Results and discussionThe physical properties of the Cu/SiO2 catalysts and SiO2 support are listed in Table 1. It was found that both the surface area and the pore volume of Cu/SiO2 catalysts decreased after the active component Cu was loaded into SiO2. Interestingly, the average pore size of AE-Cu/SiO2 was much larger than that of the support SiO2. The reason is that the silica wall was dissolved in the alkaline solution and then the dissolved silicate reacted with the neutral copper complex Cu(OH)2(H2O)4 to form copper phyllosilicate [18]. The exposed Cu surface area determined by N2O titration suggests that the AE-Cu/SiO2 has a better copper dispersion than that of WI-Cu/SiO2, and the similar result will be presented later.
|
|
Table 1 Physicochemical properties of the Cu/SiO2 catalysts and SiO2 support. Sample Surface area |
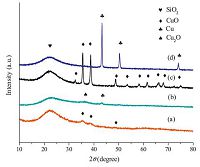
XRD patterns of the calcined and reduced Cu/SiO2 catalysts are shown in Fig. 1. The broad diffraction peak at around 2θ = 22.5° is assigned to the amorphous SiO2 in all samples. In the calcined catalysts, the colors of WI-Cu/SiO2 and AE-Cu/SiO2 were gray and dark green respectively, implying the two catalysts contained different copper species. Actually, only CuO (JCPDS 80-1916) species was detected at 2θ = 35.5, 38.7, 48.6° in the calcined Cu/ SiO2 catalysts. In the reduced catalysts, the sharp diffraction peak of metal Cu (JCPDS 89-2838) at 2θ = 43.3, 50.5, 74.1° was observed in the reduced WI-Cu/SiO2, and the weak diffraction peak of metal Cu at 2θ = 43.3° was also observed in the reduced AE-Cu/SiO2. However, a new weak diffraction peak appeared at 2θ = 36.4° in the reduced AE-Cu/SiO2 which was corresponding to Cu2O (JCPDS 05- 0667). According to the different colors and the new diffraction peak, there should exist a new copper species (copper phyllosilicate) in calcined AE-Cu/SiO2 except for CuO, and the new copper species finally was converted to Cu2O after reduction [14]. The presence of copper phyllosilicate will be verified via more information given below. In addition, it is clear that AE-Cu/SiO2 has a better copper dispersion than WI-Cu/SiO2 because the diffraction peaks of former catalyst were obviously lower and broader than that of latter.
|
Download:
|
| Fig. 1.XRD patterns of the calcined and reduced Cu/SiO2 catalysts: (a) the calcined AE-Cu/SiO2; (b) the reduced AE-Cu/SiO2; (c) the calcined WI-Cu/SiO2; (d) the reduced WI-Cu/SiO2. | |
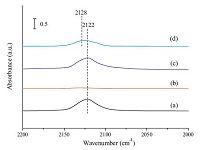
In order to confirm the existence of copper phyllosilicate, the FT-IR spectra of calcined catalysts and pure SiO2 are illustrated in Fig. S1 in Supporting information. The new peak appearing in the spectrum of AE-Cu/SiO2 at 670 cm-1 is absent both in the spectrum of WI-Cu/SiO2 and pure SiO2, which is a characteristic band of copper phyllosilicate [18]. In addition, in situ FT-IR spectra of CO species adsorbed on the reduced catalysts was used to identify the chemical adsorption state, and the results are shown in Fig. 2. The intense absorption bands near 2122 cm-1 were observed both in WI-Cu/SiO2 and AE-Cu/SiO2 before evacuation, which was assigned to CO adsorbed on Cu species (CO-Cu0 and CO-Cu+). However, the band near 2122 cm-1 in WI-Cu/SiO2 was disappeared whereas a new band formed near 2128 cm-1 after evacuation for 20 min. The new band at 2128 cm-1 was attributed to CO-Cu+ because CO could be strongly adsorbed on Cu+ while CO was weakly adsorbed on Cu0 during evacuation [19, 20].
|
Download:
|
| Fig. 2.In situ FT-IR spectra of CO species adsorbed on the reduced catalysts: (a) WICu/ SiO2: before evacuation; (b) WI-Cu/SiO2: after evacuation for 20 min; (c) AE-Cu/ SiO2: before evacuation; (d) AE-Cu/SiO2: after evacuation for 20 min. | |
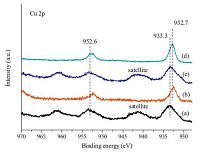
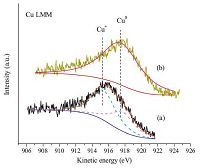
The XPS and Auger spectra (AES) of the Cu/SiO2 catalysts were carried out to identify surface valence state of copper species. As shown in Fig. 3, two obvious and intensive photoelectron peaks appearing at 952.6 eV and 932.7 eV are assigned to the Cu 2p1/2 and Cu 2p3/2 binding energy [6], respectively. The results suggest that the high valence state Cu2+ species in calcined WI-Cu/SiO2 and AE-Cu/SiO2 are effectively reduced to Cu+ and Cu0 because the characteristic satellite peaks of Cu2+ species at about 11 eV higher than 932.7 eV (Cu 2p3/2) have disappeared and the peaks at 933.3 eV are shifted to 932.7 eV [21, 22], which is consistent with the results of XRD. Considering that the Cu+ and Cu0 have almost the same binding energy of Cu 2p3/2, Cu LMM XAES is traditionally used to distinguish these two valence states by different kinetic energy [23]. According to the Fig. 4, an asymmetric and broad kinetic energy peak is observed in the reduced AE-Cu/SiO2, indicating the existence of more than one copper species. In contrast, the reduced WI-Cu/SiO2 catalyst only possesses one symmetric kinetic energy peak which is assigned to Cu0 species. As is reported in paper, the symmetric peaks at 915.2 eV and 917.7 eV are attributed to Cu+ and Cu0 [24], respectively. Through deconvolution of Cu LMM curve for the reduced AE-Cu/SiO2, the result is shown in Fig. 4 and the intensity ratio of Cu+/Cu0 is given in Table 1.
|
Download:
|
| Fig. 3.XPS spectra of the Cu/SiO2 catalysts: (a) the calcined WI-Cu/SiO2; (b) the reduced WI-Cu/SiO2; (c) the calcined AE-Cu/SiO2; (d) the reduced AE-Cu/SiO2. | |
|
Download:
|
| Fig. 4.Cu LMM Auger spectra of the reduced Cu/SiO2 catalysts: (a) AE-Cu/SiO2; (b) WI-Cu/SiO2. | |
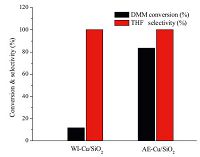
The catalytic performances of Cu/SiO2 catalysts prepared by different methods are illustrated in Fig. 5. It is clear that the DMM conversion of AE-Cu/SiO2 is much higher than that of WI-Cu/SiO2 with the same THF selectivity of 100%, the THF yield is listed in Table 1. The corresponding explanation is due to the AE-Cu/SiO2 has higher exposed Cu surface area listed in Table 1. Meanwhile, it is found that the WI-Cu/SiO2 (only Cu0) can catalyze the reaction individually, which means Cu0 is the active site. TOF value is an important standard to measure the performance of catalysts, and it refers to the reactant molecules consumed per mol active surface copper per hour. To further get insight the effect of new species of Cu+ formed in activated AE-Cu/SiO2, TOF value is calculated to eliminate the influence of the dispersion of copper and the results are listed in Table 1. Obviously, TOF value of AE-Cu/SiO2 is higher than that of WI-Cu/SiO2. The wide difference of TOF values can be attributed to the formation of Cu+ in AE-Cu/SiO2, while only Cu0 species is present at WI-Cu/SiO2. Based on the above FT-IR spectra of chemisorbed CO, the Cu+ can stabilize carbonyl compound via CO-Cu+. Moreover, it may also function as Lewis acid sites to polarize the C=O [6]. Thus, the Cu+ can improve the catalytic performance as another active site. However, researches reported that the catalytic activity would come down when the ratio of Cu+/ Cu0 exceeded an appropriate value [25, 26, 27], indicating that Cu+ is not the main active site. Based on above analysis, we concluded that Cu0 could be the main active site and the synergistic effect between Cu0 and Cu+ could further improve the activity.
|
Download:
|
| Fig. 5.The catalytic performances of Cu/SiO2 catalysts prepared by different methods at 513 K, 5 MPa, LHSV of 0.9 h-1 and H2/DMM molar ratio of 60. | |
The influence of Cu+ in Cu/SiO2 catalysts was investigated by the comparison of TOF value of the WI-Cu/SiO2 and AE-Cu/SiO2. The Cu+ and Cu0 species were generated in the activated AE-Cu/SiO2, while only Cu0 was formed in the activated WI-Cu/SiO2. The characterization suggested that the two species Cu+ and Cu0 were originated from the copper phyllosilicate and CuO during the calcination process, respectively. The dispersion of copper species affects the catalytic performance significantly, which was remarkably different between WI-Cu/SiO2 and AE-Cu/SiO2 illustrated in XRD graph. To have a single investigation of the effect of Cu+ species, the TOF values of two catalysts were calculated for eliminating the interference of the dispersion of copper species. Activated AE-Cu/SiO2 (contain Cu+ and Cu0) gave a higher TOF value than that of activated WI-Cu/SiO2 (only Cu0), which was attributed to strong adsorption between Cu+ and the C=O bond of DMM. The correlation between surface valence state of copper species and TOF values suggested that Cu0 was the main active site and the synergistic effect between Cu+ and Cu0 could further improve the activity.
AcknowledgmentFunding for the present study from the National Key Basic Research Program of China (973 Program, No. 2011CB710800) and National Natural Science Foundation of China (No. NSFC- 21406199).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.04.031.
| [1] | S.P. Müller, M. Kucher, C. Ohlinger, B. Kraushaar-Czarnetzki, Extrusion of Cu/ZnO catalysts for the single-stage gas-phase processing of dimethyl maleate to tetrahydrofuran, J. Catal. 218(2003) 419-426. |
| [2] | T. Haas, B. Jaeger, R. Weber, S.F. Mitchell, C.F. King, New diol processes:1,3-propanediol and 1,4-butanediol, Appl. Catal. A. 280(2005) 83-88. |
| [3] | W. Reppe, E. Keyssner, Production of alkinols, US Patent2232867,1941. |
| [4] | C. Ohlinger, B.Z. Czarnetzki, Improved processing stability in the hydrogenation of dimethyl maleate to γ-butyrolactone 1,4-butanediol and tetrahydrofuran, Chem. Eng. Sci. 58(2003) 1453-1461. |
| [5] | G. Centi, F. Trifiro, J.R. Ebner, et al., Mechanistic aspects of maleic anhydride synthesis from C4 hydrocarbons over phosphorus vanadium oxide, Chem. Rev. 88(1988) 55-80. |
| [6] | L.F. Chen, P.J. Guo, M.H. Qiao, et al., Cu/SiO2 catalysts prepared by the ammoniaevaporation method:texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol, J Catal. 257(2008) 172-180. |
| [7] | S.R. Wang, L.J. Zhu, Y.Y. Zhu, X.L. Ge, X.B. Li, Effects of ethanol on the in situ synthesized Cu/SiO2 catalyst:texture, structure, and the catalytic performance in hydrogenation dimethyl oxalate to ethylene glycol, Chin. Chem. Lett. 22(2011) 362-365. |
| [8] | L. Lin, P.B. Pan, Z.F. Zhou, et al., Cu/SiO2 catalysts prepared by the sol-gel method for hydrogenation of dimethyl oxalate to ethylene glycol, Chin. J. Chem. 32(2011) 957-969. |
| [9] | D.S. Brands, E.K. Poels, A. Bliek, Ester hydrogenolysis over promoted Cu/SiO2 catalysts, Appl. Catal. A. 184(1999) 279-289. |
| [10] | Y.N. Wang, X.P. Duan, J.W. Zheng, et al., Remarkable enhancement of Cu catalyst activity in hydrogenation of dimethyl oxalate to ethylene glycol using gold, Catal. Sci. Technol. 2(2012) 1637-1639. |
| [11] | Y. Huang, H. Ariga, X.L. Zheng, et al., Silver-modulated SiO2-supported copper catalysts for selective hydrogenation of dimethyl oxalate to ethylene glycol, J. Catal. 307(2013) 74-83. |
| [12] | C. Wen, A.Y. Yin, Y.Y. Cui, et al., Enhanced catalytic performance for SiO2-TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate, Appl. Catal. A 458(2013) 82-89. |
| [13] | C.S. Chen, C.C. Chen, C.T. Chen, et al., Synthesis of Cu nanoparticles in mesoporous silica SBA-15 functionalized with carboxylic acid groups, Chem. Commun. 47(2011) 2288-2290. |
| [14] | J.L. Gong, H.R. Yue, Y.J. Zhao, et al., Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites, J. Am. Chem. Soc. 134(2012) 13922-13925. |
| [15] | F. Li, C.S. Lu, X.N. Li, The effect of the amount of ammonia on the Cu0/Cu+ ratio of Cu/SiO2 catalyst for the hydrogenation of dimethyl oxalate to ethylene glycol, Chin. Chem. Lett. 25(2014) 1461-1465. |
| [16] | A.Y. Yin, X.Y. Guo, W.L. Dai, K.N. Fan, The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol:new insights on the synergetic effect between Cu0 and Cu+, J. Phys. Chem. C 113(2009) 11003-11013. |
| [17] | S. Sato, R. Takahashi, T. Sodesawa, K.I. Yuma, Y. Obata, Distinction between surface and bulk oxidation of Cu through N2O decomposition, J. Catal. 196(2000) 195-199. |
| [18] | T. Toupance, M. Kermarec, J.F. Lambert, et al., Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption, J. Phys. Chem. B 106(2002) 2277-2286. |
| [19] | K. Hadjiivanov, H. Knözinger, FTIR study of CO and NO adsorption and coadsorption on a Cu/SiO2 catalyst:probing the oxidation state of copper, Phys. Chem. Chem. Phys. 3(2001) 1132-1137. |
| [20] | T. Tsoncheva, Tz. Venkov, M. Dimitrov, C. Minchev, K. Hadjiivanov, Coppermodified mesoporous MCM-41 silica:FTIR and catalytic study, J. Mol. Catal. A. 209(2004) 125-134. |
| [21] | X.L. Zheng, H.Q. Lin, J.W. Zheng, X.P. Duan, Y.Z. Yuan, Lanthanum oxide-modified Cu/SiO2 as a high-performance catalyst for chemoselective hydrogenation of dimethyl oxalate to ethylene glycol, ACS Catal. 3(2013) 2738-2749. |
| [22] | S. Zhao, H.R. Yue, Y.J. Zhao, et al., Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2:enhanced stability with boron dopant, J. Catal. 297(2013) 142-150. |
| [23] | G.G. Jernigan, G.A. Somorjai, Carbon monoxide oxidation over three different oxidation states of copper:metallic copper, copper (I) oxide, and copper (II) oxide-a surface science and kinetic study, J Catal. 147(1994) 567-577. |
| [24] | Z.W. Huang, F. Cui, H.X. Kang, et al., Highly dispersed silica-supported copper nanoparticles prepared by precipitation-gel method:a simple but efficient and stable catalyst for glycerol hydrogenolysis, Chem. Mater. 20(2008) 5090-5099. |
| [25] | L.F. Chen, P.J. Guo, L.J. Zhu, et al., Preparation of Cu/SBA-15 catalysts by different methods for the hydrogenolysis of dimethyl maleate to 1,4-butanediol, Appl. Catal. A. 356(2009) 129-136. |
| [26] | H.R. Yue, X.B. Ma, J.L. Gong, An alternative synthetic approach for efficient catalytic conversion of syngas to ethanol, Acc. Chem. Res. 47(2014) 1483-1492. |
| [27] | B. Zhang, S.G. Hui, S.H. Zhang, et al., Effect of copper loading on texture, structure and catalytic performance of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol, J. Nat. Gas. Chem. 21(2012) 563-570. |
 2015, Vol.26
2015, Vol.26 



