Bismuth compounds are used in semiconductors,cosmetics, pigments,medicines,alloys,metallurgical additives and in the preparation and recycling of uranium nuclear fuels [1, 2]. However,a number of toxic effects on humans have been attributed to bismuth compounds,such as nephropathy,osteoarthropathy, hepatitis,and neuropathology [3]. Although multifarious techniques have been developed for bismuth determination,they require expensive instruments and high cost. So they may be prohibitive to many laboratories. Because of these considerations,potentiometric method based on ion-selective electrodes seems attractive for the determination of bismuth in several samples.
A few electroactive materials were used for the development of Bi(III) PVC membrane selective electrodes [4, 5, 6, 7, 8]. And they require improvement as they show some limitations such as narrow working ranges and interference from various metals such as K+, Hg2+,Fe3+ and Pb2+. Therefore,efforts are still needed to develop more selective and sensitive electrodes. In this research,a new ionophore 5-(3,4,5-trimethoxyphenyl)-4-amino-1,2,4-triazole- 3-thiol (L) is introduced. 1,2,4-Triazole and their derivatives are the most important types of nitrogen heterocyclic compounds. 1,2,4-Triazole-3-thiol containing sulphur and nitrogen donor atoms are known to form stable complexes with metal ions [9, 10]. We wish to prepare a highly selective and sensitive Bi(III) sensor based on L as an excellent ion carrier for monitoring Bi(III) ions in real samples.
2. ExperimentalIonophore L was synthesized according to the procedures described in the literatures [11, 12]. 2-Nitrophenyloctyl ether (o-NPOE) and potassium tetrakis(4-chlorophenylborate) (KTpClPB) were purchased from Fluka. Dibutyl phthalate (DBP),dioctyl phthalate (DOP),bis(2-ethylhexyl) sebacate (DOS),sodium tetraphenylborate (NaTPB),tetrahydrofuran (THF) (dried by sodium and distilled prior to use),poly(vinylchoride) (PVC) of high molecular mass were obtained from Shanghai Chemical Company.
Membrane solution was prepared by dissolving various membrane ingredients viz. ionophore,PVC,plasticizers (DOS, DBP,DOP and o-NPOE) and anionic excluders (NaTPB and KTpClPB) in 5 mL THF. The solution was poured into an 18 mm diameter glass ring and the solvent was allowed to evaporate at room temperature for two days. The resulting membrane was cut into small diameter disks and was sealed onto the end of the Ag/AgCl electrode barrel with a 5 wt% THF solution of PVC. Prior to potentiometric measurements,the electrodes were conditioned in a 0.01 mol/L Bi3+ solution for 24 h.
Potentials and pH values were measured by using an ion meter of model pXSJ-216 (Leici Instruments Corporation,Shanghai) and a pH meter of model 6071 (JENCO Electronics,LTD,Shanghai), respectively. The UV-vis spectra were recorded on a UV-vis spectrophotometer (Model TU-1900,Purkinje General).
3. Results and discussionUV-spectroscopic experiments were first carried out to investigate the interaction of the ionophore with various ions to selective the target ion. The absorption spectrum of L in acetonitrile (5.0 × 10-5 mol/L) exhibits two significant bands at 218 and 263 nm. Compared with other metal ions,upon addition 20 mL 1.0 × 10-2 mol/L Bi3+ ion in the solution of L,the substantial increase in absorbance and significant hyperchromic and bathochromic shift of the absorption band at 218 nm,together with the hypochromatic shift of the absorption band at 263 nm were found (Fig. S1 in Supporting information). The results suggest the preferred coordination of the Bi3+ ion by the carrier and ligand L is expected to act as a selective ionophore for the preparation of Bi3+ electrode. The formation constant of the complex between Bi3+ and ligand L,evaluated from the absorbance-mole ratio data was found to be 2.88 × 105 L/mol.
The membrane composition is important to the selectivity and sensitivity of the electrode [13, 14]. Thus the membranes based on L were optimized and the results showed that the electrode containing 0.5 wt% L,66.3 wt% o-NPOE and 33.2 wt% PVC displayed excellent potentiometric response characteristics toward Bi3+ ion ranging from 5.0 × 10-7 mol/L to 1.0 × 10-2 mol/L with a detection limit of 8.3 × 10-8 mol/L and a slope of 19.8 ± 0.5 mV/decade (Table S1 in Supporting information).
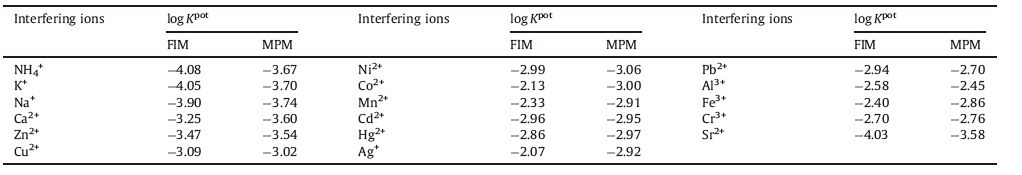
Selectivity coefficients of the electrode toward different cationic species were evaluated by using matched potential method (MPM) [15, 16] and fixed interference method (FIM) [17]. The resulting selectivity coefficient values summarized in Table 1 show that the selectivity coefficient for different interfering metal ions are sufficiently smaller than -2.0,indicating that the present sensor is significantly selective to Bi3+ ion over other ions.
|
|
Table 1 Selectivity coefficients of the bismuth (III) ion electrode. |
The pH response profile of the proposed electrode for 1.0 × 10-5 mol/L and 1.0 × 10-4 mol/L Bi3+ solutions was investigated over the pH range of 1.0-7.0. The pH was adjusted by using HNO3 and NaOH solutions. Apparently,the potential remained comparatively constant in the pH range of 3.0-6.0 (Fig. S2 in Supporting information),which may be taken as the working pH range for the sensor. The observed potential increase at lower pH values could be due to the interference of H+ ions. While at higher pHs the formation of some hydroxyl complexes of Bi3+ ions may be responsible for a decrease in potential responses.
The working of the sensor in partially non-aqueous media was also investigated using methanol-water mixtures because real samples,especially industrial effluents,may contain non-aqueous contents. The membrane worked satisfactorily in mixtures having a maximum of 10% (v/v) non-aqueous content. Above it,slope and working concentration range of the electrode was reduced and potentials showed a drift.
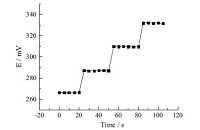
The dynamic response time of the sensor was measured by changing the concentration of Bi(III) from 1.0 × 10-6 mol/L to 1.0 × 10-3 mol/L. The resulting data depicted in Fig. 1 showed that the time needed to reach a potential within ±1.0 mV of the final equilibriumvalue after successive addition of Bi3+ ion solutions (each having a ten fold difference in concentration) was 6 s. In the reproducibility study,the electrode was dipped alternatively 10 times into 1.0 × 10-5 mol/L and 1.0 × 10-4 mol/L Bi3+ solutions. The potential readings showed standard deviations within 0.98 mV.
|
Download:
|
| Fig. 1.Response time curve of Bi3+-ISE. | |
To investigate the life time of the electrode,the calibration curves of proposed electrode at its optimized composition were periodically obtained for more than thirty days. During this time, the detection limit and the slope of the electrode remained almost constant.
The main performances of the proposed sensor are also compared with the previously reported Bi3+ selective electrodes [5, 6, 7, 8, 18, 19] (Table S2 in Supporting information). The features to mark are the better selectivity and response time of the proposed membrane electrode,making it slightly preferable among the options tested.
The proposed sensor was successfully used as an indicator electrode in potentiometric titration of 25.00 mL 1.0 × 10-3 mol/L Bi3+ solution with 1.0 × 10-2 mol/L EDTA at pH 4.0. The plot obtained (Fig. 2) is standard sigmoid shape which indicates that the sensor is sufficiently selective for Bi3+ and the end point corresponds to 1:1 stoichiometry of Bi3+-EDTA complex.

|
Download:
|
| Fig. 2.Potentiometric titration curve of Bi3+ solution with EDTA. | |
In order to testify the practical application of this technique,it was used to detect Bi3+ content in compound bismuth aluminate tablets. Five tablets were finely powdered. A portion of the powder, equivalent to about 0.2 g bismuth aluminate,was weighted accurately and dissolved in 20 mL mixture of concentrated H2SO4 and HNO3 (VH2SO4 : VHNO3 = 1 : 1). Then 12.5 mL 2 mol/L HNO3 was added. The resulting solution was transferred to a 250 mL volumetric flask and diluted with water. The content of bismuth in stomach medicine sample was measured using the proposed electrodes by the standard curve method and compared with the results from atomic absorption spectroscopy (AAS). The result of 8.38 × 10-4 ± 0.02 mol/L obtained by the proposed electrode,is in excellent agreement with 8.39 × 10-4 ± 0.01 mol/L determined by AAS.
4. ConclusionThe membrane electrode based on 5-(3,4,5-trimethoxyphenyl)- 4-amino-1,2,4-triazole-3-thiol (L) as an ionophore and o-NPOE as a plasticizer exhibited a Nernstian response to bismuth(III) ion over a concentration range of 5.0 × 10-7 mol/L to 1.0 × 10-2 mol/L with a slope of 19.8 mV/decade. It has fast response time of 6 s and better selectivity (log Kpot < -2.0) over a number of cations. The developed electrode can be employed as an indicator electrode in potentiometric titration and the determination of bismuth content in stomach medicine.
AcknowledgmentsThis work was supported by the Science Foundation of Henan Province (No. 142102310336).
Appendix A. Supplementary data
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.036.
| [1] | Z. Xing, J. Wang, S.C. Zhang, X.R. Zhang, Determination of bismuth in solid samples by hydride generation atomic fluorescence spectrometry with a dielectric barrier discharge atomizer, Talanta 80(2009) 139-142. |
| [2] | S. Moyano, R.G. Wuilloud, R.A. Olsina, J.A. Gá squez, L.D. Martinez, On-line preconcentration system for bismuth determination in urine by flow injection hydride generation inductively coupled plasma atomic emission spectrometry, Talanta 54(2001) 211-219. |
| [3] | S. Itoh, S. Kaneco, K. Ohta, T. Mizuno, Determination of bismuth in environmental samples with Mg-W cell-electrothermal atomic absorption spectrometry, Anal. Chim. Acta 379(1999) 169-173. |
| [4] | R. Ohzeki, T. Kambara, Properties of a membrane electrode based on liquid ion exchanger incorporated in poly(vinyl chloride) and its application to potentiometric titration of bismuth(III), J. Electroanal. Chem. Interface Electrochem. 88(1978) 85-90. |
| [5] | R.K. Mahajan, R.K. Puri, G. Bhargava, M.P. Mahajan, 1,3,4-Trisubstituted-2-azetidinone derivatives as novel receptors for bismuth (III) ion-selective electrodes:application in pharmaceutical and glass samples, Anal. Lett. 42(2009) 2444-2459. |
| [6] | S.V. Kharitonov, Polymeric membrane ion-selective electrode for determination of bismuth (III) in pharmaceutical substances, J. Pharm. Biomed. Anal. 30(2002) 181-187. |
| [7] | Z.N. Yan, S.Q. Wang, H.X. Wang, S.Y. Wu, Bismuth (III) PVC membrane ion selective electrodes based on two compounds:acylhydrazone and thiosemicarbazone with 1,3,4-thiadiazole, Mater. Sci. Eng. C 33(2013) 2562-2568. |
| [8] | L. Liu, L. Wang, H.Z. Yin, Y.J. Li, X.W. He, The preparation and application of bismuth (III) ion-selective electrode based on nanoparticles of bismuth sulfide, Anal. Lett. 39(2006) 879-890. |
| [9] | D.M.S. Paqhaleh, L. Hashemi, V. Amani, A. Morsali, A.A. Aminjanov, Synthesis of two new nano-structured mercury (II) complexes with 4-methyl-4H-1,2,4-triazole-3-thiol ligand by sonochemical method, Inorg. Chim. Acta 407(2013) 1-6. |
| [10] | R.F. Zhang, Q.F. Wang, Q.L. Li, C.L. Ma, Syntheses and characterization of triorganotin (IV) complexes of Schiff base derive from 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol and 5-amino-1,3,4-thiadiazole-2-thiol with p-phthalaldehyde, Inorg. Chim. Acta 362(2009) 2762-2769. |
| [11] | T. George, T.D. Mehta, R. Tahilramani, J. David, P.K. Talwalker, Synthesis of some striazoles with potential analgesic and antiinflammatory activities, J. Med. Chem. 14(1971) 335-338. |
| [12] | H.T. Du, H.J. Du, Synthesis and biological activity of 6-(substituted)-3-(3,4,5-trimethoxyphenyl)-1,2,4-triazolo[3,4-b] [1,3,4] thiadiazole, Chin. J. Org. Chem. 30(2010) 137-141. |
| [13] | M.B. Gholivand, Y. Mozaffari, PVC-based bis (2-nitrophenyl) disulfide sensor for zinc ions, Talanta 59(2003) 399-407. |
| [14] | W. Zhang, L. Jenny, U.E. Spichiger, A comparison of neutral Mg2+-selective ionophores in solvent polymeric membranes:complex stoichiometry and lipophilicity, Anal. Sci. 16(2000) 11-18. |
| [15] | E. Bakker, Selectivity of liquid membrane ion-selective electrodes, Electroanalysis 9(1997) 7-12. |
| [16] | Y. Umezawa, P. Buhlmann, K. Umezawa, K. Tohda, S. Amemiya, Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations (Technical Report), Pure Appl. Chem. 72(2000) 1851-2082. |
| [17] | Y. Umezawa, K. Umezawa, H. Sato, Selectivity coefficients for ion-selective electrodes:recommended methods for reporting KA,Bpot values (IUPAC technical report), Pure Appl. Chem. 67(1995) 507-518. |
| [18] | M.F. de Souza Teixeira, A.Z. Pinto, O. Fatibello-Filho, Ion-selective electrode for bismuth (III) in ethylenediamintetraacetate medium, Talanta 45(1997) 249-255. |
| [19] | D. Li, T. Sun, The development and application of the bismuth ion-modified carbon paste electrode, Phys. Testing Chem. Anal. Part B:Chem. Anal. 42(2006) 359-360. |
 2015, Vol.26
2015, Vol.26