Three-dimensional nanomaterials with controllable hierarchical structures have attracted increasing interest in nanotechnology [1],including the fields of sensoring [2],catalysis [3],electrode materials [4] and drug delivery [5]. Flower-like particle is one of the intriguing three-dimensional architectures,which resemble the morphology of flowers and exhibit high surface areas compared with common spherical particles. Most flower-like structures are fabricated from carbon materials or metal oxides [6, 7, 8, 9]. In addition,flower-like structure could also be obtained from polymeric compounds. For example,Liu et al. prepared flower-like polymer superstructures using polymer/zeolite composites with the aid of supercritical CO2 [10]. Other flower-like polymeric materials from the assembly of polyaniline [11],nucleoside [12] and cellulose stearoyl ester [13] have also been reported. So far, there are few reports on such unique morphology made from organic components. Ge et al. fabricated organic-inorganic nanoflowers using copper (II) ions as the inorganic component and various proteins as the organic component [14].
Octadecylamine (ODA),a long-chain amphiphilic alkyl amine, has been widely used in the Langmuir-Blodgett technique as a template because of its self-organization character [15],as well as in the preparation of three-dimensional architecture materials as stabilizer and structure-directing reagent [16]. Benitez et al. have studied the formation mechanism of ODA monolayer on mica and found that the monolayer existed as porous islands [17]. However, the morphology of aggregated ODA from solutions has lacked enough attention. In this letter,we report the interesting morphologies of ODA,including the specific microsphere or flower-like structure,obtained from the solutions of different solvent. Furthermore,graphene is introduced into ODA to prepare graphene/ODA composites,which also present the flower-like architecture.
2. ExperimentalGraphene oxide was prepared through a modified Hummer’s method and reduced graphene oxide (rGO) was obtained according to the literature [18]. Briefly,graphene oxide was dispersed in hydrazine (2% in water) and stirred at 80 °C for 6 h. Then the black suspension was centrifuged and the resulting rGO was thoroughly washed with water. Finally,the rGO powder was obtained by freeze-drying from a freeze dryer.
An amount of ODA was weighed and dissolved in toluene, ethanol,acetone and chloroform to prepare the corresponding solution with certain concentration,respectively. For the preparation of rGO/ODA composites,a certain amount of rGO was added to ODA solution before ultrasonic treatment of the mixture for 10 min. Then ODA solutions or rGO/ODA homogeneous suspensions were dripped on silicon wafers or glass slides and the solvents volatilized at room temperature in air for the scanning electron microscopy (SEM),contact angle,X-ray diffraction (XRD) or conductivity characterization.
3. Results and discussionOwing to the self-assembly characteristics,ODA is usually used as template in the preparation of LB films of other materials,but the morphology of ODA after solvent evaporation from its solution has not got enough attention. Only monolayers of ODA from the dilute solution have been studied [17]. We have found that due to the different solvent and concentration,the ODA morphology finally obtained after solvent evaporation exhibits different structural features. Fig. 1 shows the SEM images of ODA with different morphologies from toluene,ethanol,chloroform and acetone solutions. From 10 mg/mL toluene solution,ODA possesses a rectangular configuration in micron scale (Fig. 1a),and ODA from 10 mg/mL ethanol solution shows a surface topography of irregular wrinkles (Fig. 1b). From the low concentration (1 mg/mL) of chloroform solution,only disordered ODA fragments are obtained (Fig. 1c). When the concentration increases to 10 mg/mL,ODA sheets with the size of more than 10 mm are uniformly formed (Fig. 1d). Whereas at higher concentration, petal-like laminas emerge,which aggregate to flower-like structure (Fig. 1e). For ODA from acetone solution,the concentration of 10 mg/mL could yield the morphology of spherical and flower-like particle composed of ODA petals (Fig. 1f).

|
Download:
|
| Fig. 1.SEM images of ODA morphology from the solution of different solvent: (a) toluene (10 mg/mL), (b) ethanol (10 mg/mL), chloroform (c: 1 mg/mL; d: 10 mg/mL; e: 20 mg/mL) and (f) acetone (10 mg/mL). | |
ODA molecule has a hydrophilic amino group and a hydrophobic long alkyl chain. In the process of solvent evaporation,due to the molecular interaction between ODA and the solvents,ODA molecules assemble or aggregate into different morphologies. In addition,it could be found that from the solution of the highvolatile solvents (chloroform and acetone),ODA is apt to form the particular particle or flower-like morphology,while from the solvents with relatively low volatility (toluene and ethanol),ODA tends to form the sheet or amorphous morphology.
ODA is usually used in the preparation of two and threedimensional structural materials as a template material or structure-directing agent. Based on the above unique morphology of ODA,the functional composite materials could be prepared by combination of ODA with other materials,which might present a special structure. In this context,the composite material involved ODA and graphene is fabricated. Currently,researches related to both of them are only focused on the covalent modification of graphene with ODA [19, 20, 21]. Fig. 2 shows the morphologies of rGO/ ODA composites with mass ratio of 1:15 (rGO:ODA) prepared from the solution of chloroform and acetone. Obviously,at low magnification,the composite materials exhibit uniform and porous microspheres with ~10 μm diameter (Fig. 2a and c). While at high magnification,these spherical structures from chloroform and acetone demonstrate distinct architecture (Fig. 2b and d). The microsphere from chloroform solution is composed of compactly accumulated laminas with the thickness of ~30 nm. Whereas the rGO/ODA composite from acetone solution shows the flower-like structure,which consists of loosely assembled petals.
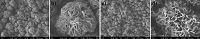
|
Download:
|
| Fig. 2.SEM images of rGO/ODA composites from chloroform (a and b) and acetone (c and d) solutions. | |
Mixed solvents were also used to prepared rGO/ODA composite and it was found that the mixed ethanol/acetone solvent could affect the morphology of the composite. The SEM images of rGO/ ODA composite from the mixed solvent are shown in Fig. 3 (ethanol/acetone volume ratios of 4:1 for Fig. 3a and 1:4 for Fig. 3b). Although ODA morphology from ethanol solution is irregular (Fig. 1b),after the addition of acetone into ethanol,the resulting rGO/ODA could obtain the flower-like shape (Fig. 3a). When the ratio of ethanol and acetone decreases to 1:4,excellent flower-like structure appears (Fig. 3b),which seems looser than the structure from acetone.

|
Download:
|
| Fig. 3.SEM images of rGO/ODA composite from ethanol/acetone solution with the v:v ratio of (a) 4:1 and (b) 1:4. (c) XRD patterns of ODA and rGO/ODA composite. (d) Contact angle measurement of rGO/ODA composite. | |
XRD was used to investigate the structure of ODA and rGO/ODA composite (mass ratio of 1:15). As shown in Fig. 3c,the patterns of the two samples demonstrate similar features,which indicates that the introduction of rGO into ODA has little affect on the assembly of ODA. Combining the SEM images,it could be confirmed that dispersed rGO is wrapped by ODA through van der Waals interaction and the composite presents the morphology of predominant ODA component as flower-like structure.
The flower-like morphology is the typical micro-nano structure, which should have good hydrophobic properties [22]. The contact angle measurement was performed to characterize the hydrophobicity of ODA and rGO/ODA composite. According to previous report,the contact angle of ODA monolayers is less than 1008 [23]. In our case,for the ODA from toluene,the contact angle is 1048, while for the ODA from ethanol with wrinkled surface,the contact angle increases to 1188. For the flower-like morphology of ODA from chloroform and acetone,the contact angle reaches 1228 and 1308,respectively,which suggests that the hierarchical structure of the surface efficiently improves the hydrophobicity of ODA. As for the flower-like rGO/ODA composite,the contact angle measurements give similar results. For instance,rGO/ODA composite with mass ratio of 1:15 from acetone performs the contact angle of 1328 (Fig. 3d).
In addition,the incorporation of rGO could afford conductivity to the insulating ODA. By four-point probe method,the conductivity of rGO/ODA with the mass ratio of 1:15 could get to 0.23 S/m, indicating that it has potential applications in sensing or field emission materials.
4. ConclusionA series of studies have been carried out to explore the morphologies of ODA from different solvents,as well as the rGO/ ODA composites. It is found that spherical flower-like ODA particles could be formed from chloroform and acetone solution. The introduction of rGO into ODA leads to rGO/ODA composite with similar hierarchical structure. XRD results indicate that the addition of rGO has little effect on the structure of ODA. Contact angle tests show that ODA and rGO/ODA composites with such unique micro-nano structure exhibit high hydrophobicity. These results might be helpful for the further research of ODA with other functional components to fabricate specific three-dimensional materials.
AcknowledgmentsThis work was supported by the Natural Science Foundation of China (Nos. 21173266 and 21473250) and the Fundamental Research Funds for the Central Universities,the Research Funds of Renmin University of China (No. 11XNJ021).
| [1] | W.L. Noorduin, A. Grinthal, L. Mahadevan, J. Aizenberg, Rationally designed complex, hierarchical microarchitectures, Science 340(2013) 832-837. |
| [2] | C.J. Martinez, B. Hockey, C.B. Montgomery, S. Semancik, Porous tin oxide nanostructured microspheres for sensor applications, Langmuir 21(2005) 7937-7944. |
| [3] | Z.H. Bao, M.R. Weatherspoon, S. Shian, et al., Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas, Nature 446(2007) 172-175. |
| [4] | Z.Y. Wang, L. Zhou, X.W. Lou, Metal oxide hollow nanostructures for lithium-ion batteries, Adv. Mater. 24(2012) 1903-1911. |
| [5] | D. Peer, J.M. Karp, S. Hong, et al., Nanocarriers as an emerging platform for cancer therapy, Nat. Nanotechnol. 2(2007) 751-760. |
| [6] | T. Nakanishi, K. Ariga, T. Michinobu, et al., Flower-shaped supramolecular assemblies:hierarchical organization of a fullerene bearing long aliphatic chains, Small 3(2007) 2019-2023. |
| [7] | X. Zhang, M. Takeuchi, Controlled fabrication of fullerene C60 into microspheres of nanoplates through porphyrin-polymer-assisted self-assembly, Angew. Chem. Int. Ed. 48(2009) 9646-9651. |
| [8] | B.I. Kharisov, A review for synthesis of nanoflowers, Recent Pat. Nanotechnol. 2(2008) 190-200. |
| [9] | Y.H. Sun, P.P. Dong, X. Lang, J.M. Nan, A novel rose flower-like SnO hierarchical structure synthesized by a hydrothermal method in an ethanol/water system, Chin. Chem. Lett. 25(2014) 915-918. |
| [10] | Y. Wang, Z.M. Liu, B.X. Han, et al., Fabrication of flowerlike polymer superstructures using polymer/zeolite composites prepared with supercritical CO2, J. Phys. Chem. B 109(2005) 2605-2609. |
| [11] | C.Q. Zhou, J. Han, R. Guo, Controllable synthesis of polyaniline multidimensional architectures:from plate-like structures to flower-like superstructures, Macromolecules 41(2008) 6473-6479. |
| [12] | H. Zhao, X.R. Guo, S.L. He, et al., Complex self-assembly of pyrimido[4,5-d]pyrimidine nucleoside supramolecular structures, Nat. Commun. 5(2014) 3108. |
| [13] | K. Zhang, A. Geissler, X.L. Chen, et al., Polymeric flower-like microparticles from self-assembled cellulose stearoyl esters, ACS Macro Lett. 4(2015) 214-219. |
| [14] | J. Ge, J.D. Lei, R.N. Zare, Protein-inorganic hybrid nanoflowers, Nat. Nanotechnol. 7(2012) 428-432. |
| [15] | Y.L. Lee, Surface characterization of octadecylamine films prepared by Langmuir-Blodgett and vacuum deposition methods by dynamic contact angle measurements, Langmuir 15(1999) 1796-1801. |
| [16] | Y.F. Xu, D.K. Ma, X.A. Chen, D.P. Yang, S.M. Huang, Bisurfactant-controlled synthesis of three-dimensional YBO3/Eu3+ architectures with tunable wettability, Langmuir 25(2009) 7103-7108. |
| [17] | J.J. Benitez, M.A. San-Miguel, S. Dominguez-Meister, J.A. Heredia-Guerrero, M. Salmeron, Structure and chemical state of octadecylamine self-assembled monolayers on mica, J. Phys. Chem. C 115(2011) 19716-19723. |
| [18] | J.L. Zou, F. Kim, Diffusion driven layer-by-layer assembly of graphene oxide nanosheets into porous three-dimensional macrostructures, Nat. Commun. 5(2014) 5254. |
| [19] | Z.Y. Lin, Y. Liu, C.P. Wong, Facile fabrication of superhydrophobic octadecylaminefunctionalized graphite oxide film, Langmuir 26(2010) 16110-16114. |
| [20] | S. Choudhary, H.P. Mungse, O.P. Khatri, Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications, J. Mater. Chem. 22(2012) 21032-21039. |
| [21] | Z.L. Chen, F.Y. Kam, V. Keerthi, et al., Efficient surfactant-free and chemical reductant-free solvothermal deoxidation of solution-processable sub-stoichiometric graphene oxide, J. Mater. Chem. C 1(2013) 7246-7254. |
| [22] | J.H. Xu, M. Li, Y. Zhao, Q.H. Lu, Advance of wetting behavior research on the superhydrophobic surface with micro- and nano-structures, Prog. Chem. 18(2006) 1425-1433. |
| [23] | K.G. Patil, V. Santhanam, S.K. Biswas, K.G. Ayappa, Combined atomic force microscopy and modeling study of the evolution of octadecylamine films on a mica surface, J. Phys. Chem. C 114(2010) 3549-3559. |
 2015, Vol.26
2015, Vol.26 


