b Oil & Gas Field Applied Chemistry Key Laboratory of Sichuan Province, Southwest Petroleum University, Chengdu 610500, China;
c Xinjiang Petroleum Survey and Design Institute, Urumqi 830026, China
Due to fast development of modern industry, the discharged industrial wastes have seriously polluted natural water, which brought great troubles to human life [1]. New requirements of World Health Organization for the permissible limits of chromium( VI) in drinking water at the level of 0.05 ppm are a prime reason for renewed interest in developing novel highly efficient technologies for sewage purification.
Absorption method has the advantages of simplicity, efficiency and excellent regeneration ability, which makes it suitable as an efficient technique for the removal of heavy metals [2, 3, 4]. The removal efficiency of Cr(VI) on solid adsorbent can be greatly improved by exploring various novel adsorbents with higher surface area to volume ratio.
Recently, there has been an increasing use of magnetic iron or iron oxide nanoparticles for water treatments because of its easy operation and recovery [1, 5, 6, 7]. To improve the dispersion stability of the nano-size iron oxides in suspension medium, surface functions of magnetic iron oxide coated with polymers or inorganic compounds has won increasing attention [8, 9, 10, 11, 12, 13, 14, 15, 16, 17]. Deposition of mesoporous carbon layers on magnetic nanoparticles is considered as an effective way to inhibit the aggregation of magnetic nanoparticles [18, 19, 20]. It has been also reported in our previous publications that mesoporous carbon layer homogenously coated on the surface of Fe3O4 could effectively inhibit the aggregation of Fe3O4 nanoparticles [21]. Additionally, it could also greatly improve the adsorption capacity of Cr(VI) from waste water due to the presence of carbon layer with porous structure.
Layered double hydroxides (LDHs), as well-known hydrotalcites structures, have been widely utilized as adsorbent for extensive applications due to their unique anion exchange capability. Moreover, the spent adsorbent can be easily regenerated and exhibit a similar adsorption capacities as the fresh LDH adsorbents. In general, LDH consists of positively charged main layers with anions located between the layers to balanced the positive layer charges, which benefited the adsorption of Cr(VI) [22, 23].
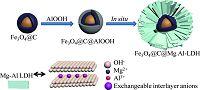
With the above-mentioned analysis, considering the combination of high ion-exchange capability of MgAl-LDH, high adsorption ability of mesoporous carbon and excellent recovery of Fe3O4 nanoparticles, a novel Fe3O4@C@MgAl-LDH adsorbent was designed and developed by the chemical self-assemble method in this work. The structural and surface properties of the prepared nano-sized Fe3O4@C@MgAl-LDH particles were studied by using a comprehensive set of characterization techniques. The adsorption capacity and adsorption kinetics of Fe3O4@C@MgAl-LDH for Cr(VI) were measured and discussed. The influences of pH, temperature and contact time on adsorption performance over Fe3O4@C@MgAl- LDH were also investigated in bath reactor for the Cr(VI) adsorption. To our best knowledge, the adsorption properties of Cr(VI) on Fe3O4@C@MgAl-LDH adsorbent have not been reported in the published literature.
2. Experimental2.1. Materials
All chemicals with AR grade and deionized water (>18.25 MΩ cm) was used to make solutions. FeCl3·6H2O, ethylene glycol, urea (CO(NH)2), D(+)-glucose, aluminum isopropoxide (Al(OPr)3), HNO3 and Mg(NO3)2·6H2O were all purchased from Sinopharm Chemical Reagent Co., China.
2.2. Synthesis of Fe3O4@C microspheresThe magnetic Fe3O4@C microspheres were prepared by a facile one-pot hydrothermal self-assembly method as reported previously [24]. Typically, 2.5 mmol of FeCl3·6H2O was dissolved into 30 mL of ethylene glycol to form a clear yellow solution under vigorous stirring. And then, 25 mmol of urea (CO(NH)2) and 0.5 mmol of D(+)-glucose were added to the above solution. After being stirred at room temperature for 30 min, the mixed homogeneous solution was transformed to a Teflon-lined stainless- steel autoclave, sealed and heated at 200 ℃ for 12 h. After the reaction completed, the obtained samples were further washed with deionized water and absolute ethanol respectively for six times. Finally, the magnetic particles were separated with external magnetic fields and then dried in muffle furnace under vacuum at 60 ℃ for 6 h (Scheme 1).
|
Download:
|
| Scheme 1.Schematic illustration for fabrication of Fe3O4@C microspheres. | |
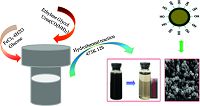
Fe3O4@C@MgAl-LDH nanoparticles were prepared by the following two procedures. Firstly, the surface of Fe3O4@C was modified by AlOOH by the sol-gel method. Secondly, the MgAl- LDH was deposited on the surface of Fe3O4@C@AlOOH. The boehmite (AlOOH) were prepared by the method reported by Wei et al. [25, 26]. Typically, aluminum isopropoxide (Al(OPr)3) (11.3 g) was dissolved in 100 mL of deionized water by stirring at 90 ℃ for 20 min. HNO3 (1.0 mol/L) was then slowly added drop wise to the solution to initiate the hydrolysis of Al(OPr)3, with the pH values of the solution being held in the range of 3-4. After stirring at 90 ℃ for 2 h, the resulting mixture was slowly cooled to atmospheric temperature, and the solid boehmite was obtained after the evaporation of water. Subsequently, the Fe3O4@C microspheres were dispersed in the AlOOH primer sol for 1 h with vigorous agitation. The resulting Fe3O4@C@AlOOH were washed with ethanol and dried in air for 30 min. The whole process (dispersion, withdrawing, drying) was repeated ten times. In the following step, 0.005 mol of Mg(NO3)2·6H2O and 0.04 mol of urea were dissolved in 100 mL of deionized water to form a solution. A designed amount of Fe3O4@C@AlOOH were immersed into the solution and then sealed and maintained at 80 ℃ for 24 h. The resulting Fe3O4@C@MgAl-LDH was further washed with water and dried in the oven at 90 ℃ for 24 h (Scheme 2).
|
Download:
|
| Scheme 2.Schematic illustration for fabrication of Fe3O4@C@MgAl-LDH. | |
The morphologies of the as-synthesized nanoparticles were obtained with FE-SEM (Hitachi S-3400FEI) with a field emission electron microscope at an accelerating voltage of 20 kV. The morphologies and particle size of prepared magnetic nanoparticles were characterized by transmission electron microscopy (TEM) with an H-600 IV (Hitachi, Japan) at an accelerating voltage of 200 kV. X-ray diffraction (XRD) patterns were recorded on a Philips X’pert PRO diffractometer using Cu Kα radiation (λ = 0.15406 nm) with an accelerating voltage of 40 kV and tube current 40 mA. Magnetic behavior was analyzed by a vibrating ample magnetometer (VSM) (MPMS-XL-7, Quantum Design Company, USA). FT-IR spectra were performed at room temperature on a Bruker Tensor 27 FT-IR spectrometer with KBr pellets. The zeta potential of asprepared particles were measured at various pH values with a Malvern Zetasizer 2000 (Malvern Instruments, UK). The TGA-DTG analyses have being carried out in an air flow of 20 cm3/min at ramping rate of 10 ℃/min with a TGA-DTG thermal analyzer (STA-449 F3, NETZSCH, Germany). The sample loading was typically 20-30 mg.
2.5. Adsorption experimentsA model waste water solution containing Cr(VI) with the concentration of 100 mg/L was prepared by dissolving a fixed quantity of potassium dichromate (K2Cr2O7) in deionized water. Batch adsorption performances of magnetic nanoparticles were carried out by mixing 0.02 g of Fe3O4@C@MgAl-LDH in 20 mL of above model waste water solution K2Cr2O7 solution in a 100 mL conical flask. The pH value of the waste water solution was adjusted by 1.0 mol/L HCl and 0.5 mol/L NaOH solutions. Effect of pH and temperature on the adsorption capacity of Cr(VI) was investigated respectively over Fe3O4@C@MgAl-LDH nanoparticles. After adsorption, the sorbents were separated with a magnet, and the concentration of Cr(VI) in the supernatant was determined, which was used to calculate desorption efficiency. When the solution became limpid, a portion of supernatant was taken, and the Cr(VI) concentration in the supernatant was determined with a spectrophotometer (UV-1800, SHIMADZU, Japan).
3. Results and discussion 3.1. Structure and morphological characterizationFig. 1(A)-(C) displays the SEM images of Fe3O4@C, MgAl-LDH and Fe3O4@C@MgAl-LDH nanoparticles, respectively. It presents the homogeneously spherical particles of Fe3O4@C, while the MgAl-LDH sample consists of a clear layer shaped particles, indicating a class of 2D-nanostructured anionic clays. Over Fe3O4@C@MgAl-LDH nanoparticles (Fig. 1(C)). MgAl-LDH with layered structure directly grows on the surface of spherical Fe3O4@C, and chemical bonding ensures a strong adhesion of MgAl-LDH to Fe3O4@C. In addition, hexagonal flake-shaped MgAl- LDH crystallites with high dispersion and regular crystal form grow in interlaced directions. Fig. 1(D)-(F) shows the TEM images of Fe3O4@C and Fe3O4@C@MgAl-LDH nanoparticles. The formation of spherical Fe3O4 nanoparticles with a size of around 100-200 nm is observed in Fig. 1(D). Meanwhile, some rough surfaces with spots are observed over Fe3O4@C, indicating that a thin carbon membrane have been successfully coated on the surface of the Fe3O4 nanoparticles. Fig. 1(E)-(F) displays the TEM images of Fe3O4@C@MgAl-LDH. As shown in Fig. 1(D), MgAl-LDH was attached and coated on the surface of spherical Fe3O4@C nanoparticles via synergic interactions. Interestingly, the Fe3O4 nanoparticles were encapsulated inside with the size of around 100-200 nm.

|
Download:
|
| Fig. 1.SEM images of (A) Fe3O4@C, (B) MgAl-LDH, (C) Fe3O4@C@MgAl-LDH. Also shown are (D) TEM image of Fe3O4@C and (E, F) TEM images of Fe3O4@C@MgAl-LDH. | |
Fig. 2(A) exhibits the Fourier transform infrared (FT-IR) spectra of the magnetite nanoparticles, carbon coated Fe3O4, MgAl-LDH and Fe3O4@C@MgAl-LDH synthesized in this study. On the FT-IR spectra of Fe3O4 (curve a), the characteristic peaks at 587 cm-1 were assigned to Fe-O in the Fe3O4 phase [11, 12, 27]. The weak bands 1622 and 1388 cm-1 corresponded to the symmetric and asymmetric stretching of C=O in the -COOH group, respectively [28], which indicates the function group -COOH coated on the surface of nano Fe3O4 [29, 30]. From curve b, the characteristics of 1614 and 3418 cm-1 were assigned to the C=C vibrations and O-H stretching, which are a result of the aromatization of glucose and the presence of numerous hydrophilic groups [27]. The characteristic adsorption band of 1365 cm-1 (curve c) was associated with the intercalation of CO3 2- anion [31]. The spectrum of Fe3O4@C@MgAl-LDH (curve d) indicates a mixture of Fe3O4@C and MgAl-LDH. The former is evidenced by the characteristic peaks of 3500 cm-1 (O-H stretching), 1614 cm-1 (C=C vibration) and 1365 cm-1 (interlayer CO3 2- vibration). It is immediately obvious that there is a satisfactory agreement between the results obtained by SEM and FT-IR.

|
Download:
|
| Fig. 2.(A) FT-IR spectra of (a) Fe3O4, (b) Fe3O4@C, (c) MgAl-LDH, (d) Fe3O4@C@MgAl-LDH. (B) XRD patterns of (i) Fe3O4, (ii) Fe3O4@C, (iii) MgAl-LDH, (iv) Fe3O4@C@MgAl-LDH. | |
Fig. 2(B) shows the XRD patterns of Fe3O4, Fe3O4@C, MgAl-LDH and Fe3O4@C@MgAl-LDH. The diffraction peaks of Fe3O4 nanoparticles at 24.68, 32.08, 35.48, 42.28, 52.78 and 62.78 are indexed as the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1) and (4 4 0) lattice planes of face-centered cubic structure of Fe3O4 phase (JCPDS card No. 65- 3107). The pattern of MgAl-LDH (curve iii) displays the XRD reflections located at the angles typical of a hydrotalcite-like phase, containing carbonate anions in the interlayer space: sharp and symmetrical for (0 0 3), (0 0 6), (1 1 0) and (1 1 3), and broad and asymmetrical for (0 0 9), (0 1 5) and (0 1 8), respectively. This result is in agreement with previous XRD data reported by Cavani [31] and Wagenknecht et al. [31, 32]. Besides the LDH diffraction peaks, the XRD pattern of Fe3O4@C@MgAl-LDH (curve iv) exhibits the clear reflections of Fe3O4 phase with low intensity compared with Fe3O4@C. This suggests that the lower crystallinity of the iron oxides particles in Fe3O4@C@MgAl-LDH samples due to the LDH modification.
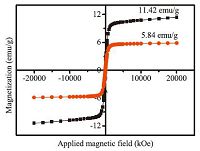
Fig. 3 presents the magnetization curves of Fe3O4@C and Fe3O4@C@MgAl-LDH. The magnetization curve intensity of Fe3O4@C@MgAl-LDH is much lower than that of Fe3O4@C. By quantitative calculation, the saturation magnetizations of Fe3O4@C@MgAl-LDH is 5.84 emu/g, while it is 11.42 emu/g for Fe3O4@C, suggesting that the introduction of MgAl-LDH leads to lower saturation magnetization. However, separation of the Fe3O4@C@MgAl-LDH from heavy metal ion solutions is still easily completed in a few seconds with permanent hand-held magnets.
|
Download:
|
| Fig. 3.Magnetization curves of prepared samples at room temperature. (a) Fe3O4@C and (b) Fe3O4@C@MgAl-LDH. | |
The influence of contact time on the adsorption of Cr(VI) over as-prepared Fe3O4, Fe3 O4@C and Fe3O4@C@MgAl-LDH samples were investigate(Fig. S1 in Supporting information). The adsorption properties varied with contact time exhibits three areas. At the beginning of 30 min, the adsorption rate climb very sharply over three samples and then keep slow increase until it arrives at adsorption equilibrium at about 60 min.
Relatively low equilibrium adsorption capacity is observed over pure Fe3O4 nanoparticles. The equilibrium adsorption capacity of Fe3O4@C@MgAl-LDH attains a value of 120.5 mg/g, which is 13 times higher than with the Fe3O4. The order of three nanoparticles absorbent tested here in equilibrium adsorption capacity is following: Fe3O4@C@MgAl-LDH > Fe3O4@C > Fe3O4. The high adsorption efficiency of Fe3O4@C@MgAl-LDH for Cr(VI) could be attributed to the synergic function of physical and chemical adsorption.
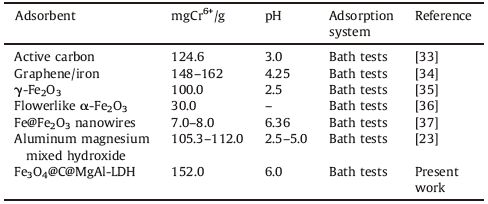
Adsorption capacities of some sorbents reported in the literature are summarized briefly in Table 1. By comparison, the novel Fe3O4@C@MgAl-LDH prepared here show fast adsorption and high adsorption capacity.
|
|
Table 1 Comparison of the maximum adsorption capacities for Cr(VI) removal over various adsorbents. |
The effect of pH on the removal of Cr(VI) is investigated by testing values of pH ranging from 2.0 to 10.0 at a temperature of 20 ℃ and for concentrations of Cr(VI) 2 mg/g. The contact time has been kept 60 min so as to arrive at adsorption equilibrium (Fig. 4(A)). As expected, an equilibrium adsorption capacity of Fe3O4@C@MgAl-LDH much depends on the pH of solution treated. The adsorption capacity firstly climbs very sharply at pH range from 2 to 6, and then attaints a maximum value (143.3 mg/g) at the pH of 6. While the equilibrium adsorption capacity of Fe3O4@C@MgAl- LDH for Cr(VI) gradually decreases with the increase of pH from 6 to 12. Similar trends also were observed in previous publications reported by Li et al. [23]. The Fe3O4@C@MgAl-LDH nanoparticles exhibit the optimal adsorption capacity at the pH 6. As mentioned by above zeta potentials analysis (see Fig. S2 in Supporting information), the surface of Fe3O4@C@MgAl-LDH assembles a large number of positive charges at pH 6 (lower than PZC 8.8), thereby driving the diffusion of dichromate ions (Cr2O7 2-) and their subsequent adsorption on the Fe3O4@C@MgAl-LDH nanoparticles.

|
Download:
|
| Fig. 4.(a) Effect of pH value on the adsorption of Fe3O4@C@MgAl-LDH for Cr(VI) (T = 20 ℃; m = 0.02 g); (b) the equilibrium adsorption of Cr(VI) was as a function of temperature on Fe3O4@C@MgAl-LDH (pH 6; m = 0.02 g). | |
The effect of temperature on the adsorption capacity of Cr(VI) is investigated at six constant temperatures 15 ℃, 20 ℃, 30 ℃, 40 ℃, 50 ℃ and 60 ℃. The amount of Cr(VI) removed on the Fe3O4@C@MgAl-LDH adsorbents is plotted versus the temperature as shown in Fig. 4(B). The temperature going from 15 ℃ to 40 ℃, the equilibrium adsorption capacity for Cr(VI) increases from 120.5 mg/g to 152.0 mg/g, and attained a maximum value at 40 ℃. While for temperature higher than 40 ℃, the equilibrium adsorption capacity for Cr(VI) decreases quite rapidly to 129.3 mg/g at the temperature of 60 ℃. As previous publication reported by Daneshvar et al. [37], the temperature could yield two main effects on the adsorption process. An increase in temperature is known to increase the diffusion rate of the adsorbate molecules across the external boundary layer and within the pores. This could be the result of decreasing solution viscosity. Furthermore, changing the temperature will modify the equilibrium capacity of the adsorbent for a particular adsorbate.
3.3. Adsorption kinetics and thermodynamicsThe sorption dynamics of Cr2O7 ions to Fe3O4@C@MgAl-LDH were evaluated by adding 0.02 g of the as-obtained Fe3O4@C@MgAl- LDH into 100 mL of a solution containing 0.1 mg/L each of the metal ions (pH 6.0) at different temperature. Fig. 5 depicts the evolution of the sorption data of Cr(VI) on Fe3O4@C@MgAl-LDH as function of time at the temperatures ranging from 15 ℃ to 40 ℃, the initial pH being fixed to its optimal value of pH 6 for all the experiments. From Fig. 6, it appears a rapid initial rise of the adsorption capacities Qt and the equilibrium is almost reached within 60 min.

|
Download:
|
| Fig. 5.Equilibrium time (a) and second-order kinetic equation (b) for adsorption of Cr(VI) on Fe3O4@C@MgAl-LDH at different temperature (pH 6.0). | |

|
Download:
|
| Fig. 6.Performance of Fe3O4@C@MgAl-LDH by six regeneration cycle. | |
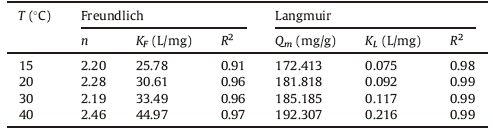
Considering the high values of the regression coefficient R2 near unity in Table 2, it is clear that the adsorption data fits the Langmuir models much better than Freundlich models at our four chosen temperatures. As it can be seen from Table 2, the near unity regression coefficient values (R2) related to the Langmuir model indicate that its good fit to Cr(VI) adsorption equilibrium data. Table 2 also gives values of n significantly higher than 1. The situation n > 1 is the most common and corresponds to an L-type of normal Langmuir isotherm [38]. Furthermore, Table 2 indicates that the Langmuir parameter Qm and the Freundlich parameter KF increases with temperature increasing, suggesting that the enhancement of temperature facilitated Cr(VI) capacity adsorption.
|
|
Table 2 Influence of temperature on values of Freundlich and Langmuir isotherm constants. |
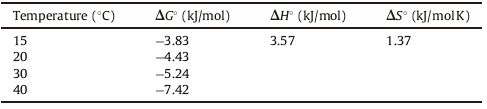
To investigate the whether the sorption of Cr(VI) on the Fe3O4@C@LDH composite in the presence is endothermic or exothermic, the thermodynamic parameters, namely, standard enthalpy (ΔH°), standard entropy(ΔS°), and standard free energy (ΔG°) was calculated at various temperatures using the following equations:
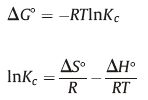
|
|
Table 3 Thermodynamic parameters for Cr(VI) adsorption at pH 6 over Fe3O4@C@MgAl-LDH nanoparticles. |
The validity of the kinetic models is tested by the magnitude of the regression coefficient R2, given in Table 4. It is important to note that for a pseudo first-order model, the correlation coefficient is always less than 0.98, which is indicative of a not good correlation. Therefore, the pseudo-first-order model is not suitable for modeling the adsorption of Cr(VI) over Fe3O4@C@MgAl-LDH. In contrast, the application of a pseudo-second-order model leads to much better regression coefficients, all higher than 0.99. Thus, the pseudo-second-order kinetic model is well suitable to model the sorption curves of Cr(VI) over Fe3O4@C@MgAl-LDH.
|
|
Table 4 Rate adsorption constants for three kinetic models at different temperature and pH 6.0. |
Fig. 5(b) shows that the rate of adsorption increases with increasing temperatures. A similar trend was observed by Hamadi et al. [39] for the adsorption of Cr(VI) on activated carbon. Previous results in the literature showed that the kinetics of adsorption is strongly dependent on the type of adsorbent material. Hamadi et al. [39] showed that a pseudo-second-order kinetic equation is well suited for modeling the sorption kinetics of Cr(VI) onto various adsorbents. However, Singh et al. [40] have shown a better performance of the pseudo-first-order kinetics model in the case of the adsorption of lead and chromium from aqueous solutions by red mud.
In summary, the adsorption of Cr(VI) onto the tested Fe3O4@C@MgAl-LDH is well described by the Langmuir model regardless of the experimental conditions (i.e. temperature of solution). As well known that the Langmuir equation assumes a homogenous surface, an accurate fitting means the active sites are certainly homogeneously distributed on the surface of adsorbent tested. The theory of Langmuir is actually based on the fixation of a monolayer of adsorbate molecules on the pores surface. In addition, Qe values calculated from the linear plots of t/qt versus t for pseudo-second-order kinetic equation showed a good agreement with experimental Qe values. Consequently, it can be confirmed that the adsorption of Cr(VI) onto Fe3O4@C@MgAl-LDH obeys a pseudo-second-order reaction rate.
3.4. Recovery test for Cr(VI) removalIt is of great significance to evaluate the repeatability of Fe3O4@C@MgAl-LDH in use and the possibility of removing Cr(VI). 0.02 g used Fe3O4@C@MgAl-LDH were stirred with (50 mL) aqueous solution (pH 6.0 adjusted by NaOH 1 mol/L) at room temperature for 1 h to desorb the Cr(VI). The final Cr(VI) concentration in the aqueous phase was determined by using a spectrophotometer. The adsorption of Cr(VI) is possible by controlling pH. 0.01 mol/L NaOH was the most effective for desorption of Cr(VI) reported by Irene Lo et al. [2]. Thereafter, the absorbent were washed with 0.01 mol/L NaOH eluent and neutralized water until neutralized pH and dried at 60 ℃ for 2 h and again subjected to adsorption processes to determine the reusability of the Fe3O4@C@MgAl-LDH. Adsorption-desorption cycles were repeated six times by using the same Fe3O4@C@MgAl- LDH.
4. ConclusionMagnetic Fe3O4@C@MgAl-LDH nanoparticles is an effective adsorbent for the removal of Cr(VI) from aqueous solutions. Adsorption capacity of Cr(VI) much depend on pH of solutions and the best results are obtained at the pH range from 6.0 to 8.0. The maximum adsorption capacity doubly increased as the temperature increased from 20 ℃ to 50℃. The experimental data fitted well to the pseudo first-order kinetic model. The Langmuir isotherm provided the best correlation for Cr(VI) onto the Fe3O4@C@MgAl-LDH nanoparticles. Adsorption capacity calculated according to the Langmuir isotherm was 170 mg/g at an initial pH of 1.0 for the 1000 mg/L Cr(VI) solution. Thermodynamic parameters were evaluated and the adsorption is endothermic showing monolayer adsorption of Cr(VI).
AcknowledgmentsThis work was supported by Scientific Research Starting Project of SWPU (No. 2014QH013), Open Fund of state key laboratory of Oil & Gas reservoir geology and exploitation (No. PLN 1126), and major cultivation project of Sichuan Provincial Department of Education Science and Technology Achievement Transformation (No. 14CZ0005).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015. 05.026.
| [1] | J. Hu, G. Chen, I. Lo, Selective removal of heavy metals from industrial wastewater using maghemite nanoparticle:performance and mechanisms, J. Environ. Eng. 132(2006) 709-715. |
| [2] | V.K. Gupta, I. Ali, T.A. Saleh, A. Nayak, S. Agarwal, Chemical treatment technologies for waste-water recycling-an overview, RSC Adv. 2(2012) 6380-6388. |
| [3] | V.K. Gupta, T.A. Saleh, Sorption of pollutants by porous carbon, carbon nanotubes and fullerene-an overview, Environ. Sci. Pollut. Res. 20(2013) 2828-2843. |
| [4] | V.K. Gupta, S. Agarwal, T.A. Saleh, Synthesis and characterization of aluminacoated carbon nanotubes and their application for lead removal, J. Hazard. Mater. 185(2011) 17-23. |
| [5] | J. Hu, G. Chen, I.M.C. Lo, Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles, Water Res. 39(2005) 4528-4536. |
| [6] | J. Hu, I.M.C. Lo, G. Chen, Removal of Cr(VI) by magnetite nanoparticle, Water Sci. Technol. 50(2004) 139-146. |
| [7] | K. Chen, G.H. Wang, W.B. Li, et al., Application of response surface methodology for optimization of Orange II removal by heterogeneous Fenton-like process using Fe3O4 nanoparticles, Chin. Chem. Lett. 25(2014) 1455-1460. |
| [8] | Y.L. Lei, F. Chen, Y.J. Luo, L. Zhang, Three-dimensional magnetic graphene oxide foam/Fe3O4 nanocomposite as an efficient absorbent for Cr(VI) removal, J. Mater. Sci. 49(2014) 4236-4245. |
| [9] | S.X. Zhang, Y.Y. Zhang, J.S. Liu, et al., Thiol modified Fe3O4@SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal,, Chem. Eng. J. 226(2013) 30-38. |
| [10] | W. Jiang, W.F. Wang, B.C. Pan, et al., Facile fabrication of magnetic chitosan beads of fast kinetics and high capacity for copper removal, ACS Appl. Mater. Interfaces 6(2014) 3421-3426. |
| [11] | K. Zargoosh, H. Abedini, A. Abdolmaleki, M.R. Molavian, Effective removal of heavy metal ions from industrial wastes using thiosalicylhydrazide-modified magnetic nanoparticles, Ind. Eng. Chem. Res. 52(2013) 14944-14954. |
| [12] | F. Ge, M.M. Li, H. Ye, B.X. Zhao, Effective removal of heavy metal ions Cd2+, Zn2+, Pb2+, Cu2+ from aqueous solution by polymer-modified magnetic nanoparticles, J. Hazard. Mater. 211-212(2012) 366-372. |
| [13] | J.F. Liu, Z.S. Zhao, G.B. Jiang, Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water, Environ. Sci. Technol. 42(2008) 6949-6954. |
| [14] | W. Yantasee, C.L. Warner, T. Sangvanich, et al., Removal of heavy metals from aqueous systems with thiol functionalized superparamagnetic nanoparticles, Environ. Sci. Technol. 41(2007) 5114-5119. |
| [15] | Y.H. Deng, D.W. Qi, C.H. Deng, X.M. Zhang, D.Y. Zhao, Superparamagnetic highmagnetization microspheres with an Fe3O4@SiO2 core and perpendicularly aligned mesoporous SiO2 shell for removal of microcystins, J. Am. Chem. Soc. 130(2007) 28-29. |
| [16] | M. Bhaumik, A. Maity, V.V. Srinivasu, M.S. Onyango, Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite, J. Hazard. Mater. 190(2011) 381-390. |
| [17] | Y.Q. Wang, B.F. Zou, T. Gao, et al., Synthesis of orange-like Fe3O4/PPy composite microspheres and their excellent Cr(VI) ion removal properties, J. Mater. Chem. 22(2012) 9034-9040. |
| [18] | Z.Y.Zhang, J.L.Kong,Novelmagnetic Fe3O4@Cnanoparticles as adsorbents for removal of organic dyes from aqueous solution, J. Hazard. Mater. 193(2011) 325-329. |
| [19] | S.C. Han, L.F. Hu, Z.Q. Liang, et al., One-step hydrothermal synthesis of 2D hexagonal nanoplates of α-Fe2O3/Graphene composites with enhanced photocatalytic activity, Adv. Funct. Mater. 24(2014) 5719-5727. |
| [20] | K. Cheng, Y.M. Zhou, Z.Y. Sun, et al., Synthesis of carbon-coated, porous and waterdispersive Fe3O4 nanocapsules and their excellent performance for heavy metal removal applications, Dalton Trans. 41(2012) 5854-5861. |
| [21] | H. Zhang, D.L. Liu, L.L. Zeng, M. Li, b-Cyclodextrin assisted one-pot synthesis of mesoporous magnetic Fe3O4@C and their excellent performance for the removal of Cr(VI) from aqueous solutions, Chin. Chem. Lett. 24(2013) 341-343. |
| [22] | K.H. Goh, T.T. Lim, Z.L. Dong, Application of layered double hydroxides for removal of oxyanions:a review, Water Res. 42(2008) 1343-1368. |
| [23] | Y.J. Li, B.Y. Gao, T. Wu, et al., Hexavalent chromium removal from aqueous solution by adsorption on aluminum magnesium mixed hydroxide, Water Res. 43(2009) 3067-3075. |
| [24] | J. Zheng, Z.Q. Liu, X.S. Zhao, et al., One-step solvothermal synthesis of Fe3O4@C core-shell nanoparticles with tunable sizes, Nanotechnology 23(2012) 165601. |
| [25] | Y.F. Zhao, S. He, M. Wei, D.G. Evans, X. Duan, Hierarchical films of layered double hydroxides by using a sol-gel process and their high adaptability in water treatment, Chem. Commun. 46(2010) 3031-3033. |
| [26] | M. Shao, F. Ning, J. Zhao, et al., Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins, J. Am. Chem. Soc. 134(2011) 1071-1077. |
| [27] | M.Y. Zhu, G.W. Diao, Magnetically recyclable Pd nanoparticles immobilized on magnetic Fe3O4@C nanocomposites:preparation, characterization, and their catalytic activity toward Suzuki and Heck coupling reactions, J. Phys. Chem. C 115(2011) 24743-24749. |
| [28] | M. Răcuciu, Synthesis protocol influence on aqueous magnetic fluid properties, Curr. Appl. Phys. 9(2009) 1062-1066. |
| [29] | Y. Sahoo, A. Goodarzi, M.T. Swihart, et al., Aqueous ferrofluid of magnetite nanoparticles:fluorescence labeling and magnetophoretic control, J. Phys. Chem. B 109(2005) 3879-3885. |
| [30] | R. Xu, H.C. Zeng, Synthesis of nanosize supported hydrotalcite-like compounds CoAlx(OH)2+2x(CO3)y(NO3)x-2y·nH2Oon γ-Al2O3, Chem. Mater. 13(2001) 297-303. |
| [31] | F. Cavani, F. Trifiro`, A. Vaccari, Hydrotalcite-type anionic clays:preparation, properties and applications,, Catal. Today 11(1991) 173-301. |
| [32] | F.R. Costa, A. Leuteritz, U. Wagenknecht, et al., Intercalation of Mγ-Al layered double hydroxide by anionic surfactants:Preparation and characterization, Appl. Clay Sci. 38(2008) 153-164. |
| [33] | K. Selvi, S. Pattabhi, K. Kadirvelu, Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon, Bioresour. Technol. 80(2001) 87-89. |
| [34] | H. Jabeen, V. Chandra, S. Jung, et al., Enhanced Cr(VI) removal using iron nanoparticle decorated graphene, Nanoscale 3(2011) 3583-3585. |
| [35] | C.Y. Cao, J. Qu, W.S. Yan, et al., Low-cost synthesis of flowerlike α-Fe2O3 nanostructures for heavy metal ion removal:adsorption property and mechanism, Langmuir 28(2012) 4573-4579. |
| [36] | Z.H. Ai, Y. Cheng, L.Z. Zhang, J.R. Qiu, Efficient removal of Cr(VI) from aqueous solution with Fe@Fe2O3 core-shell nanowires, Environ. Sci. Technol. 42(2008) 6955-6960. |
| [37] | N. Daneshvar, D. Salari, S. Aber, Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake, J. Hazard. Mater. 94(2002) 49-61. |
| [38] | K. Fytianos, E. Voudrias, E. Kokkalis, Sorption-desorption behaviour of 2,4-dichlorophenol by marine sediments, Chemosphere 40(2000) 3-6. |
| [39] | N.K. Hamadi, X.D. Chen, M.M. Farid, M.G.Q. Lu, Adsorption kinetics for the removal of chromium(VI) from aqueous solution by adsorbents derived from used tyres and sawdust, Chem. Eng. J. 84(2001) 95-105. |
| [40] | V.K. Singh, P.N. Tiwari, Removal and recovery of chromium(VI) from industrial waste water, J. Chem. Technol. Biotechnol. 69(1997) 376-382. |
 2015, Vol.26
2015, Vol.26