b Shanghai Key Laboratory of Advanced Polymeric Materials, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
Conjugated polymers have been greatly investigated because of its unique optical and electric properties which can be attributed to its extensively delocalized p-electron networks [1, 2, 3, 4, 5]. Their delocalized backbone make their absorption and emission properties very sensitive to the environmental perturbation, whichmake them become progressive candidates for various biological and chemical chemosensors [6, 7, 8, 9, 10, 11, 12]. Polydiacetylenes (PDAs) are very interesting materials among conjugated polymers [13, 14, 15, 16, 17, 18, 19]. PDAs are readily prepared by UV irradiation of self-assembled diacetylene supramolecules without need of any initiators or catalysts. Consequently, there are no unwanted by-product and impurity in the resulting polymer.Well-designedDAmonomer couldbe easilypolymerizedin aqueous solution inthe formof nanostructured liposomes or vesicles because of their amphiphilic property. The PDAs could go through colorimetric transition caused by the external stimuli such as heat (thermochromism) [20, 21, 22, 23], solvent (solvatochromism) [24, 25, 26], mechanical stress(mechanochromism) [27, 28, 29]andligand-receptor (affinochromism) [9, 30, 31, 32] interaction.
Many investigations have been made on the thermochromism of PDAs. Although some PDA system displayed reversible thermochromism, but in most of the cases reported earlier, reversible thermochromism was observed only in the narrow temperature range, for example 25-55 °C [33], or they were not fully reversible as they deteriorate to violet to red rather than blue to red after first heating-cooling cycle [34]. In the investigation described below, we prepared a new polydiacetylene, (Bip-PDA), in which biphenylcarboxylic acid group is connected in its side chain, the biphenyl group provides strong aromatic interaction between monomers and carboxylic acid induces intermolecular H-bonding during self-assembly. The new polydiacetylene displays completely reversible thermochromism in the range of 20-95 °C in solution.
2. Experimental 2.1. Materials and equipmentsUnless otherwise noted, materials were obtained from commercial suppliers and were used without further purification. Flash chromatography was carried out on silica gel (200-300 mesh). The laser light scattering particle size measurements were performed on a ALV/CGS-5022F. 1H NMR and 13C NMR spectra were recorded using Bruke AVANCE 500 MHz. Mass spectra were obtained using a Micromass GCTTM Time-of-Flight Mass Spectrometer. UV irradiation was performed by LUYOR LEC-180L hand lamp. Probe sonication was carried out by using Fisher Sonic dismembrator model 550. UV absorption spectra were obtained on UVIKON 933 Double Beam UV-vis Spectrometer.

|
Download:
|
| Fig. 1.Size distribution of PDA sol obtained from laser light scattering. | |
Oxalyl chloride (0.61 mL, 7.15 mmol) was added dropwise to a methylene chloride solution containing 0.702 g 1.87 mmol) of 10, 12-pentacosadiynoic acid (PCDA) at room temperature. The resulting mixture was stirred for 30 min and then one drop of fresh DMF was added as catalyst. Above solution was stirred at room temperature for another 5 h. Residue obtained by concentration of the above solution dissolved in 20 mL THF, then added dropwise a solution containing 0.403 g (1.88 mol) 4'-hydroxy-4-biphenylcarboxylic acid and 0.4 mL (2.89 mmol) of triethylamine. The resulting solution was stirred overnight at room temperature under N2. The solvent was removed under vacuum and the residue purified by silica gel column chromatography (CH2Cl2 100%) to give the desired diacetylene monomer, Bip-DA 0.93 g, 87%), as a white solid. ESI-MS: m/z [1TD$DIF]570.3709 [M]+. 1H NMR (500 MHz, CDCl3) δ 8.16 (d, 2H, J = 8.2 Hz), 7.65 (d, 2H, J = 8.3 Hz, ), 7.62 (d, 2H, J = 8.5 Hz), 7.17 (d, 2H, J = 8.5 Hz), 2.56 (t, 2H, J = 7.4 Hz), 2.22 (m, 4H), 1.79-1.71 (m, 2H), 1.53-1.45 (m, 4H), 1.42-1.19 (m, 26H), 0.85 (t, 3H, J = 6.9 Hz). 13C NMR (126 MHz, CDCl3) δ 172.17, 170.53, 150.85, 145.43, 137.41, 130.64, 128.27, 128.03, 127.00, 122.04, 77.55, 65.26, 65.13, 34.30, 31.82, 29.60, 29.54, 29.24, 28.99, 28.94, 28.79, 28.76, 28.25, 28.19, 24.79, 22.58, 19.10, 14.01.
2.3. Preparation and polymerization of vesiclesThe diacetylene monomer Bip-DA (11.4 mg, 0.02 mmol) was dissolved in a small amount of DMF (1 mL) and the clear solution was added to 19 mL hot deionized water dropwise to make a 1.0 mmol/L of dispersed monomer suspension. The resultant suspension was probe-sonicated at 80 °C for 25 min and the resulting solution was filtered by a 0.8 mm filter and the filtrate cooled at 4 °C for 12 h. Polymerization was performed at room temperature by irradiating the solution with 254 nm UV light (1 mW/cm2) for 15 min.
The sizes of the lipid assembled vesicles within the PDA sol were characterized by dynamic light scattering (DLS) technique. The DLS size distribution revealed that the average hydrodynamic diameter of the particles was 153.6 nm (Fig. 1).
2.4. Colorimetric response (CR) valueIn order to determine thermochromic properties of the polydiacetylene we synthesized, (Bip-PDA), all UV spectroscopy measurements were carried out using a 1 cm optical path length cell. The blue polymer was first transformed to its red phase by using a heating-cooling cycle. UV-vis spectra were recorded during the first cycle. To quantify the extent of the blue-to-red transitions within the polymer, the % colorimetric response (CR) value was calculated using the following equation:


|
Download:
|
| Fig. 2.Photographs of as aqueous solution of Bip-PDA at 20 °C, 55 °C, and 95 °C. | |
Bip-DA was prepared using the route shown in Scheme 1. Firstly, oxalyl chloride was treated with 10, 12-pentacosadiynoic acid (PCDA) to get its acid chloride derivative. The acid chloride was then reacted with 4'-hydroxy-4-biphenylcarboxylic acid to generate Bip-DA in 87% yield after the silica column chromatography. The synthesized monomer Bip-DA was fully characterized by 1H NMR, 13C NMR and ESI Mass spectra. These characterizations confirmed the formation of Bip-DA. The Bip-DA monomers were then transformed into vesicles by a routine procedure. UV irradiation of self-assembled monomers Bip-DA for 15 min resulted in the formation of stable and blue-colored PDA sol.
3.2. Thermochromism of Bip-PDA in solutionFig. 2 shows images of a vial containing an aqueous solution of Bip-PDA at various temperatures. The DA solution shows completely reversible colorimetric change in the range of 20-95 °C during heating-cooling cycles. Unlike previously reported PDAs which display thermochromic changes over relatively narrow temperature ranges or initially they undergo color changes that deteriorate to violet (or purple) to red rather than blue to red after the first cycle. Fig. 3(a) shows UV absorption spectrum of PDA solution at different temperature during heating and cooling procedures. At 20 °C, the PDA solution shows the typical blue color corresponding to a visible absorption maximum at 633 nm. When the temperature is raised from 20 °C to 95°C, the PDA solution became red and absorption maximum of the solution undergoes a gradual blue shift to 539 nm. When the temperature cooled down to 20 °C, the absorption maximum shifts back to 630 nm and the original intensity is recovered. The gradual shift of absorption maxima without isosbestic points observed with PDA supramolecules indicate that numerous intermediate states exist during the cycle. This observation is very important for the understanding of the mechanism of reversible PDA thermochromism. The CR value was obtained from the visible spectra recorded for PDA solution. During the heating process from 20 °C to 95 °C, the CR value was increased gradually and reach its maximum in 95 °C, indicate that the transformation from blue to red color with temperature rising. When cooled down to 20 °C, the red PDA solution returns to the blue with CR value decreasing. The Bip-PDA has a complete colorimetric reversibility and thermal stability as repeating the thermal cycles more than 20 times without color bleaching (Scheme 2).

|
Download:
|
| Scheme 1.Synthesis of Bip-DA. | |

|
Download:
|
| Fig. 3.(a) UV–vis spectra of PDA sols during heating–cooling cycle. (b) Colorimetric response value of the Bip-PDA solution to cycles of temperature changes in the range of 20–95 °C. | |
Previous study [35, 36, 37] indicated that head group interactions (hydrogen bonding, aromatic interactions, etc.) are essential for performing reversible colorimetric thermochromism. Molecular orientation of the methylene chains between backbone and head group are formed in a distorted state during the polymerization process. Thus, the release of the mechanical strain upon thermal stimulation results in C-C bond rotation of the polymer backbone and decreasing of effective conjugation length. The gradual blue shift of the absorption maxima without isosbestic points suggest that a number of intermediate states exist with decreasing effective conjugation length. The energy barriers between these states are small and therefore the original blue state conformation can be recovered when the thermal stimulation is removed. In this work, we introduced biphenyl group and carboxylic acid into the PDA. The carboxylic acid make the monomer Bip-DA amphiphilic and help vesicle formation in aqueous solution, and also provide hydrogen bonding between head group. The biphenyl group brings significantly strong π-π interaction between side chains. As the phenyl analog of Bip-PDA was investigated by Kim and coworkers [35] and showed a irreversible thermochromism, the enhanced aromatic interaction offered by biphenyl may play a crucial role in governing reversible thermochromism of PDA solution, which is also identical with the study of Yoon [37].
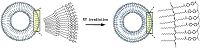
|
Download:
|
| Scheme 2.The self-assembly and polymerization of Bip-DA. | |
In the study above, a new PDA which include biphenylcarboxylic acid as head group was synthesized and its thermochromic property was studied. The Bip-PDA solution displayed complete reversibility in the colorimetric transition from blue-to-red as the temperature is varied between 20 °C and 95 °C. The strong aromatic interaction induced by biphenyl may play a significant role in performing reversible thermochromism.
AcknowledgmentsThis research was supported by National High Technology Research and Development Program of China (863 program) ([13TD$DIF]No. 2009AA035002) and the Fundamental Research Funds for the Central Universities ([13TD$DIF]No. 22A201514002).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015.05.027.
| [1] | X.R. Duan, L.B. Liu, F.D. Feng, S. Wang, Cationic conjugated polymers for optical detection of DNA methylation, lesions, and single nucleotide polymorphisms, Acc. Chem. Res. 43(2010) 260-270. |
| [2] | S. Günes, H. Neugebauer, N.S. Sariciftci, Conjugated polymer-based organic solar cells, Chem. Rev. 107(2007) 1324-1388. |
| [3] | F. Hide, M.A. Díaz-García, B.J. Schwartz, A.J. Heeger, New developments in the photonic applications of conjugated polymers, Acc. Chem. Res. 30(1997) 430-436. |
| [4] | X.L. Feng, L.B. Liu, S. Wang, D.B. Zhu, Water-soluble fluorescent conjugated polymers and their interactions with biomacromolecules for sensitive biosensors, Chem. Soc. Rev. 39(2010) 2411-2419. |
| [5] | H.N. Kim, Z.Q. Guo, W.H. Zhu, J. Yoon, H. Tian, Recent progress on polymer-based fluorescent and colorimetric chemosensors, Chem. Soc. Rev. 40(2011) 79-93. |
| [6] | X.Q. Chen, G.D. Zhou, X.J. Peng, J. Yoon, Biosensors and chemosensors based on the optical responses of polydiacetylenes, Chem. Soc. Rev. 41(2012) 4610-4630. |
| [7] | S.W. Thomas III, G.D. Joly, T.M. Swager, Chemical sensors based on amplifying fluorescent conjugated polymers, Chem. Rev. 107(2007) 1339-1386. |
| [8] | X.M. Sun, T. Chen, S.Q. Huang, L. Li, H.S. Peng, Chromatic polydiacetylene with novel sensitivity, Chem. Soc. Rev. 39(2010) 4244-4257. |
| [9] | D.H. Charych, J.O. Nagy, W. Spevak, M.D. Bendnarski, Direct colorimetric detection of a receptor-ligand interaction by a polymerized bilayer assembly, Science 261(1993) 585-588. |
| [10] | A. Herland, O. Inganäs, Conjugated polymers as optical probes for protein interactions and protein conformations, Macromol. Rapid Commun. 28(2007) 1703-1713. |
| [11] | S. Okada, S. Peng, W. Spevak, D. Charych, Color and chromism of polydiacetylene vesicles, Acc. Chem. Res. 31(1998) 229-239. |
| [12] | C.H. Fan, K.W. Plaxco, A.J. Heeger, High-efficiency fluorescence quenching of conjugated polymers by proteins, J. Am. Chem. Soc. 124(2002) 5642-5643. |
| [13] | D.J. Ahn, J.-M. Kim, Fluorogenic polydiacetylene supramolecules:immobilization, micropatterning, and application to label-free chemosensors, Acc. Chem. Res. 41(2008) 805-816. |
| [14] | D. Day, H. Ringsdorf, Polymerization of diacetylene carbonic acid monolayers at the gas-water interface, J. Polym. Sci. Polym. Lett. Ed. 16(1978) 205-210. |
| [15] | Y. Okawa, M. Aono, Nanoscale control of chain polymerization, Nature 409(2001) 683-684. |
| [16] | R. Jelinek, S. Kolusheva, Polymerized lipid vesicles as colorimetric biosensors for biotechnological applications, Biotechnol. Adv. 19(2001) 109-118. |
| [17] | O. Endo, H. Ootsubo, N. Toda, et al., Phase transition of a single sheet of sashlike polydiacetylene atomic sash on a solid surface, J. Am. Chem. Soc. 126(2004) 9894-9895. |
| [18] | J.M. Schnur, B.R. Ratna, J.V. Selinger, et al., Diacetylenic lipid tubules:experimental evidence for a chiral molecular architecture, Science 264(1994) 945-947. |
| [19] | J. Song, J.S. Cisar, C.R. Bertozzi, Functional self-assembling bolaamphiphilic polydiacetylenes as colorimetric sensor scaffolds, J. Am. Chem. Soc. 126(2004) 8459-8465. |
| [20] | D.J. Ahn, E.H. Chae, G.S. Lee, et al., Colorimetric reversibility of polydiacetylene supramolecules having enhanced hydrogen-bonding under thermal and pH stimuli, J. Am. Chem. Soc. 125(2003) 8976-8977. |
| [21] | S. Dei, T. Shimogaki, A. Matsumoto, Thermochromism of polydiacetylenes containing robust 2D hydrogen bond network of naphthylmethylammonium carboxylates, Macromolecules 41(2008) 6055-6065. |
| [22] | S.Y. Lee, Y.Y. Cho, B.U. Ye, et al., Unprecedented colorimetric responses of polydiacetylenes driven by plasma induced polymerization and their patterning applications, Chem. Commun. 50(2014) 12447-12449. |
| [23] | S.Y. Lee, J.Y. Lee, H.N. Kim, M.H. Kim, J.Y. Yoon, Thermally reversible polydiacetylenes derived from ethylene oxide-containing bisdiacetylenes, Sens. Actuators B 173(2012) 419-425. |
| [24] | C.A. Sandstedt, C.J. Eckhardt, M.J. Downey, D.J. Sandman, Optical, calorimetric, and mass spectroscopic study of nonthermochromic crystalline forms of the polydiacetylene bis(ethylurethane)-5, 7-dodecadiyne-1, 12-diol (ETCD), Chem. Mater. 6(1994) 1346-1350. |
| [25] | R.R. Chance, Chromism in polydiacetylene solutions and crystals, Macromolecules 13(1980) 396-398. |
| [26] | A.D. Nava, M. Thakur, A.E. Tonelli, Carbon-13 NMR structural studies of a soluble polydiacetylene, poly(4BCMU), Macromolecules 23(1990) 3055-3063. |
| [27] | Y. Tomioka, N. Tanaka, S. Imazeki, Effects of side-group interactions on pressureinduced chromism of polydiacetylene monolayer at a gas-water interface, Thin Solid Films 179(1989) 27-31. |
| [28] | R.W. Carpick, D.Y. Sasaki, A.R. Burns, First observation of mechanochromism at the nanometer scale, Langmuir 16(2000) 1270-1278. |
| [29] | R.A. Nallicheri, M.F. Rubner, Investigations of the mechanochromic behavior of poly(urethane-diacetylene) segmented copolymers, Macromolecules 24(1991) 517-525. |
| [30] | C.G. Wang, Z.F. Ma, Colorimetric detection of oligonucleotides using a polydiacetylene vesicle sensor, Anal. Bioanal. Chem. 382(2005) 1708-1710. |
| [31] | A. Reichert, J.O. Nagy, W. Spevak, D. Charych, Polydiacetylene liposomes functionalized with sialic acid bind and colorimetrically detect influenza virus, J. Am. Chem. Soc. 117(1995) 829-830. |
| [32] | Q. Cheng, R.C. Stevens, Coupling of an induced fit enzyme to polydiacetylene thin films:colorimetric detection of glucose, Adv. Mater. 9(1997) 481-483. |
| [33] | C. Phollookin, S. Wacharasindhu, A. Ajavakom, et al., Tuning down of color transition temperature of thermochromically reversible bisdiynamide polydiacetylenes, Macromolecules 43(2010) 7540-7548. |
| [34] | X.Q. Chen, J.Y. Yoon, A thermally reversible temperature sensor based on polydiacetylene:synthesis and thermochromic properties, Dyes Pigments 89(2011) 194-198. |
| [35] | J.-M. Kim, J.S. Lee, H. Choi, D. Sohn, D.J. Ahn, Rational design and in-situ FTIR analyses of colorimetrically reversible polydiacetylene supramolecules, Macromolecules 38(2005) 9366-9376. |
| [36] | L. Yu, S.L. Hsu, A spectroscopic analysis of the role of side chains in controlling thermochromic transitions in polydiacetylenes, Macromolecules 45(2012) 420-429. |
| [37] | S.Y. Lee, J.S. Lee, J.Y. Yoon, et al., Construction and molecular understanding of an unprecedented, reversibly thermochromic bis-polydiacetylene, Adv. Funct. Mater. 24(2014) 3699-3705. |
 2015, Vol.26
2015, Vol.26 


