Organoselenium chemistry has developed as an important synthetic tool in the hands of synthetic chemists since the discovery of the selenoxide elimination in the early 1970s [1, 2, 3, 4, 5]. Because of their synthetic applications [6] and biological activities such as antitumor,antibacterial activities and other properties [7, 8, 9, 10, 11, 12, 13, 14],selenium compounds have been increasing in importance in recent years. The introduction of organolseleno groups into organic molecules has been widely studied,in which the oxyselenenylation reaction is a very useful procedure for the anti-l,2-addition of an organylseleno group and an oxygen substituent (HO,RO,RCO2) to an olefin [15, 16, 17, 18]. In the electrophilic addition,the most common selenenylating reagents PhSeX (X = Br, Cl) are usually commercially available,or they are also prepared from oxidative cleavage of diphenyl diselenide by halogens [19]. However,due to the toxic and moisture-sensitive nature of PhSeX,nucleophilic halide anions are sometimes responsible for some undesirable processes such as addition of the halide ion and the decrease in stereoselectivity. To avoid the above drawbacks, some novel alternative reagents such as PhSeO2CCH3,PhSeO2CCH3, N-phenylselenophtalimide and N-phenylselenosuccinimide have been developed [20, 21, 22, 23]. Since diphenyl diselenide is less expensive and less toxic,a much simpler way for formation of the electrophilic phenylselenium cation is oxidation of diphenyl diselenide with oxidants such as ammonium peroxydisulfate,mnitrobenzenesulfonyl peroxide,Pb(OAc)4,Ce(NH4)2(NO3)6 and Cu2+(Cu+)/O2 systems [24, 25, 26, 27, 28, 29, 30]. However,the metal oxidants also have toxicity and some oxidations require long reaction times or high reaction temperatures,which limit the applications. Therefore, the discovering of novel and convenient experimental conditions to carry out the cleavage of Se-Se bond is a really important target.
Recently,wehave investigated the newacetoxyselenenylation of alkenes with a catalytic amount of hypervalent iodine reagent. We found that when the hypervalent iodine reagent was replaced by inorganic haloid salts combined with the oxidant m-chloroperbenzoic acid (mCPBA),the acetoxyselenenylation of alkenes was carried out smoothly and efficiently with high regioselectivity and good yields under mild conditions. In order to find cheaper and more environment-friendly catalysts,we have focused our attention on bromides and chlorides. Herein,we wish to report a novel and convenient acetoxyselenenylation of alkenes with diselenides and mCPBAusing widely available KBr orNaCl as catalyst,and to the best of our knowledge,this electrophilic addition of the in situ generated reactive electrophilic selenium species PhSeX (X = Br,Cl) to alkenes has not been previously reported.
2. ExperimentalA typical procedure for the catalytic acetoxyselenenylation of alkenes using KBr or NaCl as catalyst includes:In AcOH (AR,99.5%,1.5 mL),alkene 1a (0.6 mmol),diselenide 2a (0.2 mmol),KBr (0.08 mmol) and mCPBA (0.4 mmol) were added successively. The suspension mixture was vigorously stirred at r.t. for 3 h. Upon completion,the reaction was quenched by addition of sat. aq Na2S2O3 (2 mL),sat. aq Na2CO3 (8 mL) and H2O (5 mL). The mixture was extracted with CH2Cl2 (3×5 mL) and the combined organic phase was dried over anhydrous Na2SO4,filtered,and concentrated under reduced pressure. The residue was then purified by TLC technique (4:1 (v/v) petroleum ether/ethyl acetate) to furnish 2- acetoxy-1-selenenylation compounds 3a [31] in 90% yield.
Colorless oil; 1H NMR (500 MHz,CDCl3): δ 7.52 (dd,2H,J = 6.5, 2.9 Hz),7.39-7.30 (m,5H),7.30-7.24 (m,3H),5.96 (dd,1H,J = 8.0, 5.7 Hz),3.40 (dd,1H,J = 12.9,8.0 Hz),3.25 (dd,1H,J = 12.9,5.7 Hz), 2.04 (s,3H); 13C NMR (125 MHz,CDCl3): δ170.0,139.4,133.1, 129.8,129.1,128.5,128.4,127.2,126.6,75.2,33.4,21.0; IR (film, cm-1): v 3061,3033,1742,1371,1236,1020,738,698; MS (EI,m/z, %): 320 (M+,1.8),261 (100).
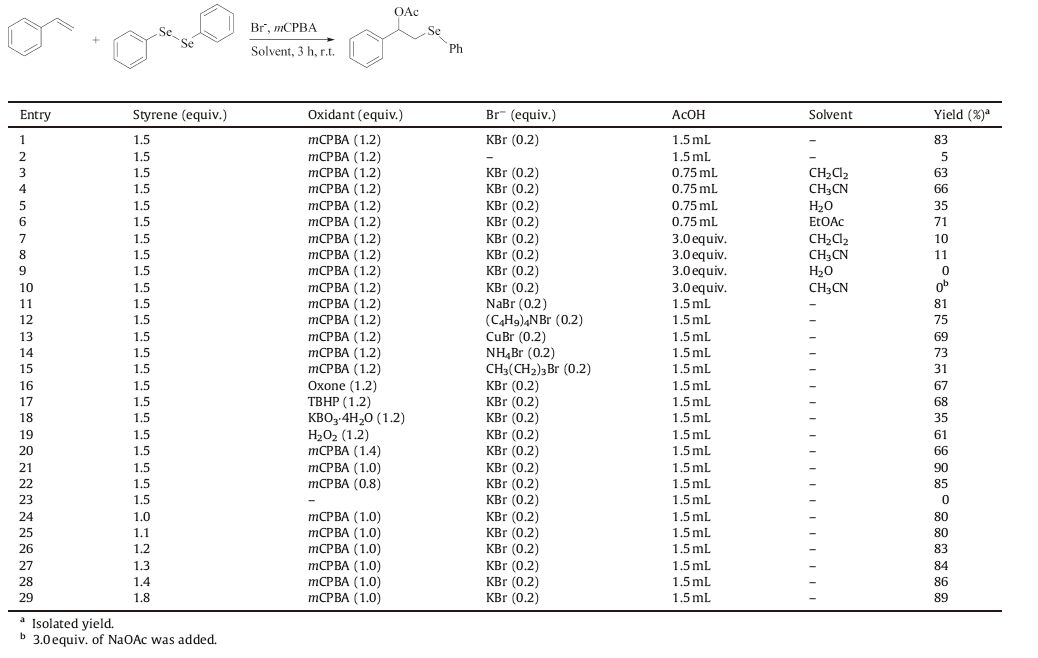
3. Results and discussionTo achieve the optimum reaction conditions,we first investigated the reaction of styrene (1a),diphenyl diselenide (2a) and oxidant mCPBA in the presence of a catalytic amount of KBr in acetic acid at r.t.When 0.2 equiv. of KBr was added to the mixture of 1.0 equiv. of 2a,1.2 equiv. of mCPBA and 1.5 equiv. of 1a in acetic acid (1.5 mL) and themixturewas stirred for 3 h,the expected addition product 1- phenyl-2-(phenylselanyl)ethyl acetate 3a was obtained in 83% yield (Table 1,entry 1). As a control experiment,the yield of 3a was observed at only 5% in the absence of KBr (entry 2). Therefore,it was obvious that KBr played a key role in the reaction. Then,the acetoxyselenenylation of styrene with diphenyl diselenide and mCPBA using 0.2 equiv. of Br- as catalyst at r.t. for 3 hwas optimized (Table 1). As shown in Table 1,with 13mmol AcOH (0.75 mL),the reaction was performed in CH2Cl2,CH3 CN,H2O and EtOAc respectively; although the amount of AcOH was reduced to half, it was also greatly in excess,but the yields were not above 71%, which meant that the reaction was more proper in neat AcOH (entries 3-6). To reproduce this,the amount of AcOHwas reduced to 3.0 equiv.,and the reaction was carried out in CH2Cl2,CH3CN and H2O respectively,only rather poor yields were determined (entries 7-9). When NaOAc was substituted for AcOH in CH3CN,no desired product was observed (entry 10). In neat AcOH,several kinds of bromide sources were studied. Among them,inorganic bromides usually gave appreciable results and KBr was the most effective while organic bromine compound led to low yield (entries 1,11-15). Compared with mCPBA,other oxidants such as Oxone®,TBHP, NaBO3▪4H2O and H2O2 usually resulted in moderate,or poor yields (entries 1,16-19). The appropriate amount of mCPBA was 1.0 equiv., and when it was absent,no productwas observed (entries 1,20-23). Finally,the optimum amount of styrene was also determined,and at 1.5 equiv.,it was the best choice (entries 21,24-29).
|
|
Table 1 Optimization of the acetoxyselenenylation of styrene using KBr as catalyst. |
In the similar model,we have also optimized the acetoxyselenenylation of styrene with diphenyl diselenide and mCPBA using 0.2 equiv. of Cl- as catalyst in AcOH at r.t. The results showed that among several chlorides such as KCl,NaCl,NH4Cl and CuCl,the most effective chloride was NaCl. The optimum amounts of styrene and mCPBA were 1.5 equiv. and 1.0 equiv.,respectively,and the proper reaction time was 5 h.
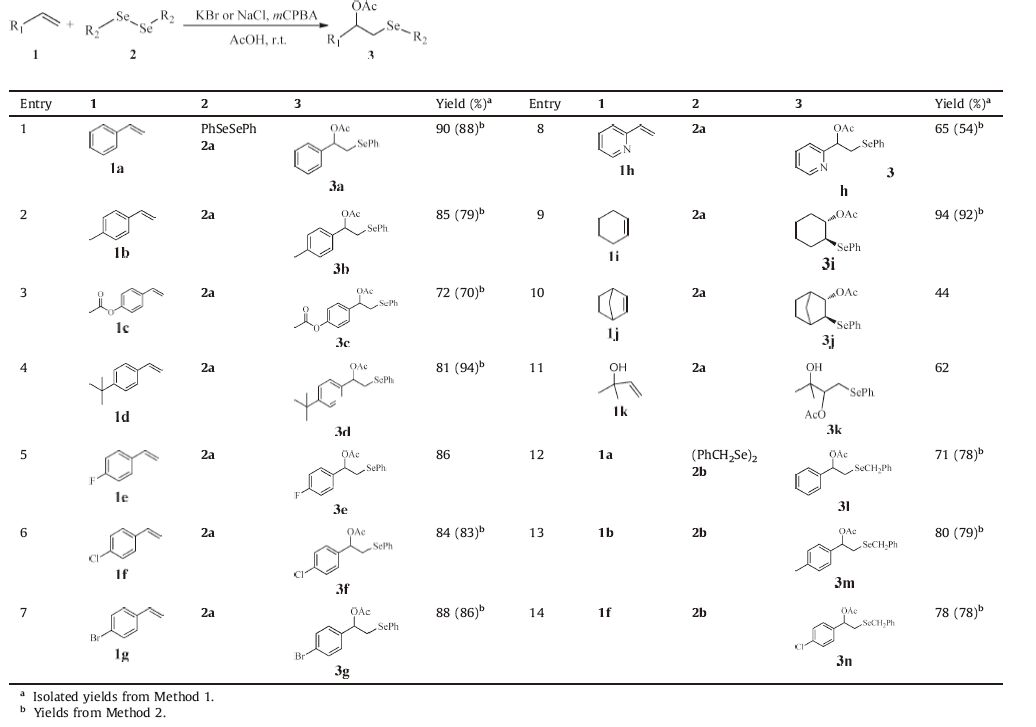
Having established the optimum conditions,the acetoxyselenenylation of 1.0 equiv. of diselenides (1),1.5 equiv. of alkenes (2) and 1.0 equiv. ofmCPBA with 0.2 equiv. of KBr in AcOH at r.t. for 3 h (Method 1),or with 0.2 equiv. of NaCl in AcOH at r.t. for 5 h (Method 2) was carried out,a series of corresponding 2-acetoxy-1- selenenylation compounds (3) were obtained; the results are summarized in Table 2.
|
|
Table 2 Preparation of 2-acetoxy-1-selenenylation compounds 3. |
As shown in Table 2,the reaction was compatible with most of the studied alkenes except bicyclo[2.2.1]hept-2-ene (1j),which provided the corresponding 2-acetoxy-1-selenenylation compounds in good to excellent yields (entries 1-14). It was also determined that the groups on the benzene ring,no matter whether they were electron-donating or electron-withdrawing groups,did not influence the yield (entries 2-7,13,14). When treated under the same conditions,the addition to cyclohexene (1i) and 1j proceeded in a trans fashion and the single stereoisomers were isolated with an excellent yield for 3i (entry 9) and a poor yield for 3j due to the steric hinderance effect of the methylene group (entry 10). Compared with 2a,the dibenzyl diselenide (2b),an aliphatic diselenide,also reacted easily with alkenes,but the yields were slightly lower (entries 12-14). From Table 2,it is obvious that the yields for most products 3 were higher when KBr was used as catalyst as opposed to NaCl,so the catalytic effect of KBr is better than NaCl in the acetoxyselenenylation of alkenes.
A proposed catalytic cycle for the KBr or NaCl mediated acetoxyselenenylation of alkenes is shown in Scheme 1. Thus,KBr is first oxidized bymCPBA to molecular bromine,which reacts with diselenide 2 to furnish the electrophilic reagent A. Then,the following an electrophilic addition of the in situ generated active ArSeBr to alkenes results in a cyclic intermediate B. After a solvolysis of B in AcOH,the desired product 2-acetoxy-1- selenenylation compound 3 as a single isomer is obtained via an SN1 mechanism for the aromatic alkenes. When aliphatic alkenes such as 1i and 1j are used as alkene substrates,the reaction provides the trans single stereoisomers via an SN2 mechanism,which supports the initial formation of the cyclic selenium intermediate B,then the acetate anion attacks the cyclic intermediate affording the corresponding anti-l,2-addition product 3. In order to validate our protocol,we checked our reaction in the absence of KBr,and only 5% yield of 3a was observed (Table 1, entry 2). We also examined the reaction of styrene with mCPBA and KBr or NaCl in acetic acid without diphenyl diselenide,and determined the addition product of Br2 or Cl2 to styrene was obtained in good yield,which supports the proposed mechanism.

|
Download:
|
| Scheme 1.Proposed mechanism for the catalyzed acetoxyselenenylation. | |
In summary,we have developed a novel and efficient catalytic procedure for the synthesis of 2-acetoxy-1-selenenylation compounds by the electrophilic addition of alkenes with diselenides and mCPBA in the presence of a catalytic amount of KBr or NaCl in AcOH at r.t. This method using widely available and less expensive KBr or NaCl as catalyst,has some advantages such as mild reaction conditions and simple procedure. Furthermore,the scope of catalytic use of inorganic haloids in acetoxyselenenylation of alkenes could be extended.
| [1] | T. Wirth, Chiral selenium compounds in organic synthesis, Tetrahedron 55(1999) 1-28. |
| [2] | T. Wirth, Organoselenium chemistry in stereoselective reactions, Angew. Chem. Int. Ed. 39(2000) 3740-3749. |
| [3] | D.M. Freudendahl, S.A. Shahzad, T. Wirth, Recent advances in organoselenium chemistry, Eur. J. Org. Chem. (2009) 1649-1664. |
| [4] | A.J. Mukherjee, S.S. Zade, H.B. Singh, et al., Organoselenium chemistry:role of intramolecular interactions, Chem. Rev. 110(2010) 4357-4416. |
| [5] | R. Walter, J. Roy, Selenomethionine, a potential catalytic antioxidant in biological systems, J. Org. Chem. 36(1971) 2561-2563. |
| [6] | G. Zeni, M.P. Stracke, C.W. Nogueira, et al., Hydroselenation of alkynes by lithium butylselenolate:an approach in the synthesis of vinylic selenides, Org. Lett. 6(2004) 1135-1138. |
| [7] | L. Letavayova, V. Vlckova, J. Brozmanova, Selenium:from cancer prevention to DNA damage, Toxicology 227(2006) 1-14. |
| [8] | P. Erkekoglu, W. Rachidi, O.G. Yuzugullu, et al., Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium, Toxicol. Appl. Pharmacol. 248(2010) 52-62. |
| [9] | H.E. Ganther, Selenium metabolism, selenoproteins and mechanisms of cancer prevention:complexities with thioredoxin reductase, Carcinogenesis 20(1999) 1657-1666. |
| [10] | A. Hartwig, H. Blessing, T. Schwerdtle, et al., Modulation of DNA repair processes by arsenic and selenium compounds, Toxicology 193(2003) 161-169. |
| [11] | P. Erkekoglu, B. Giray, W. Rachidi, et al., Effects of di(2-ethylhexyl)phthalate on testicular oxidant/antioxidant status in selenium-deficient and seleniumsupplemented rats, Environ. Toxicol. 29(2014) 98-107. |
| [12] | K. El-Bayoumy, P. Upadhyaya, V. Date, et al., Metabolism of[14C]benzyl selenocyanate in the F344 rat, Chem. Res. Toxicol. 4(1991) 560-565. |
| [13] | M.P. Rayman, Selenium in cancer prevention:a review of the evidence and mechanism of action, Proc. Nutr. Soc. 64(2005) 527-542. |
| [14] | P. Erkekoglu, M.W. Chao, W. Ye, et al., Cytoplasmic and nuclear toxicity of 3,5-dimethylaminophenol and potential protection by selenocompounds, Food Chem. Toxicol. 72(2014) 98-110. |
| [15] | A.A. Vieira, J.B. Azeredo, M. Godoi, et al., Catalytic chalcogenylation under greener conditions:a solvent-free sulfur- and seleno-functionalization of olefins via I2/DMSO oxidant system, J. Org. Chem. 80(2015) 2120-2127. |
| [16] | T.G. Back, in:S. Patai (Ed.), The Chemistry of Organic Selenium and Tellurium Compounds, vol. 2, Wiley, Chichester, 1987, pp. 115-134. |
| [17] | M. Tiecco, L. Testaferri, M. Tingoli, et al., Ring-closure reactions initiated by the peroxydisulfate ion oxidation of diphenyl diselenide, J. Org. Chem. 55(1990) 429-434. |
| [18] | M. Tiecco, L. Testaferri, M. Tingoli, et al., The reaction of diphenyl diselenide with peroxydisulphate ions in methanol a convenient procedure to effect the methoxyselenenylation of alkenes, Tetrahedron Lett. 30(1989) 1417-1420. |
| [19] | C. Paulmier, Selenium Reagents and Intermediates in Organic Synthesis, Pergamon Press, Oxford, 1986(Chapter 2). |
| [20] | K.B. Sharpless, R.F. Lauer, Electrophilic organoselenium reagents. A new route to allylic acetates and ethers, J. Org. Chem. 39(1974) 429-430. |
| [21] | H.J. Reich, Organoselenium chemistry. Benzeneselenenyl trifluoroacetate additions to olefins and acetylenes, J. Org. Chem. 39(1974) 428-429. |
| [22] | T. Hori, K.B. Sharpless, Selenium-catalyzed nonradical chlorination of olefins with N-chlorosuccinimide, J. Org. Chem. 44(1979) 4204-4208. |
| [23] | K.C. Nicolaou, D.A. Claremon, W.E. Barnette, et al., N-Phenylselenophthalimide (N-PSP) and N-phenylselenosuccinimide (N-PSS). Two versatile carriers of the phenylseleno group. Oxyselenation of olefins and a selenium-based macrolide synthesis, J. Am. Chem. Soc. 101(1979) 3704-3706. |
| [24] | M. Tiecco, L. Testaferri, M. Tingoli, et al., Production of reactivity of new organoselenium intermediates. Formation of carbon-67oxygen and carbon-67nitrogen bonds, Gazz. Chim. Ital. 126(1996) 635-643. |
| [25] | M. Yoshshida, N. Satoh, N. Kamigata, Novel method for electrophilic selenenylation using diselenide with nitrobenzenesulfonyl peroxide, Chem. Lett. (1989) 1433-1436. |
| [26] | M. Yoshshida, S. Sasage, K. Kawamura, et al., Oxidative cleavage of diselenide by m-mitrobenzenesulfonyl peroxide. Novel method for the electrophilic benzeneselenenylations of olefins and aromatic rings, Bull. Chem. Soc. Jpn. 64(1991) 416-422. |
| [27] | B.M. Trost, M. Ochiai, P.G. McDougal, Hydroxysulfenylation of olefins. An olefin cleavage with functional group differentiation, J. Am. Chem. Soc. 100(1978) 7103-7106. |
| [28] | C. Bosman, A. D'Annibale, S. Resta, et al., Oxidation of diphenyl diselenide with ceric ammonium nitrate:a Novel route for functionalization of olefins, Tetrahedron Lett. 35(1994) 6525-6528. |
| [29] | N. Miyoshi, Y. Ohno, K. Kondo, et al., Acetoxyselenation:reaction of olefins with diphenyl diselenide and cupric acetate, Chem. Lett. (1979) 1309-1312. |
| [30] | N. Taniguchi, Copper-catalyzed 1,2-hydroxysulfenylation of alkene using disulfide via cleavage of the S-S bond, J. Org. Chem. 71(2006) 7874-7876. |
| [31] | H.W. Shi, C. Yu, M. Zhu, et al., Novel and convenient acetoxyselenenylation of alkenes catalyzed by potassium iodide, J. Organomet. Chem. 776(2015) 117-122. |
 2015, Vol.26
2015, Vol.26