Quinoxaline derivatives are important components of pharmacologically active compounds, including antiviral, antibacterial, anti-inflammatory, anti-protozoal, askinase inhibitors, anticancer and anthelmintic agents [1]. In addition, quinoxaline derivatives are reported for their application in dyes, organic semiconductors, rigid subunits in macrocyclic receptors or molecular recognition, efficient electroluminescent materials, chemically controllable switches [2,3]. Generally, quinoxalines are synthesized by the condensation of aryl 1, 2-diamines with 1, 2-dicarbonyl compounds in MeOH/AcOH [4]. There are several methods for the formation of the quinoxaline ring system in the presence of various catalysts have been reported [5,6,7,8,9,10,11,12,13,14,15]. However, all these methods have some drawbacks in the light of current working performance such as application of toxic reagents, or reagents which are very expensive and less accessible, thermally unstable and formation of side products.
Nanozeolite clinoptilolite is attractive in material supports because of the excellent properties such as ease of availability of the natural, inexpensive, high specific surface area, large pore volumes, good thermal and chemical stability, and non-toxicity. Although many kinds of zeolites were used as catalysts or catalysts supports such as HB, A, NaY, X, Y and ZSM-5, the main drawbacks of these catalysts are low selectivity and using expensive or toxic materials for the synthesis of them on a large scale [16]. Acid treatment causes a modification in morphology of nanozeolite CP by the destruction of channel-blocking impurities and the development of secondary porosity [17]. Therefore, acid treatment increases the number of Si-OH groups, which can be used for the immobilization process. To explore its capability, we intended to investigate on the functionalization of natural nanozeolite clinoptilolite by propylsulfonic acid and applied as catalyst for the synthesis of quinoxalines.
2. ExperimentalAll chemicals such as 3-mercaptopropyltrimethoxysilane (MPTS), hydrogen peroxide (33 wt%) were purchased from Merck Chemical Company. The raw zeolite material was an Iranian commercial clinoptilolite (Afrandtooska Company) obtained from deposits in the region of Semnan (ca. 1 $ per kg). All the other solvents and chemicals were obtained from analytical reagent grade chemicals unless specified otherwise and purchased from Merck Company.
2.1. Synthesis of activated Nano CPThe nanozeolite clinoptilolite (5 g) was taken into a 250 mL round bottom flask and 100 mL 4 mol/L sulfuric acid was added to it. The flask mixture was refluxed for 1 h. After cooling, the supernatant was discarded and the activated nanozeolite CP was repeatedly washed with deionized water (250 mL) until the solution became neutral and finally dried in oven at 80 ℃ overnight to obtain the white solid product. The activated nanozeolite CP was designated as AT-Nano CP.
2.2. Synthesis of propylsulfonic acid functionalized AT-Nano CP (NZ-PSA)AT-Nano CP (2 g) was taken into a 50 mL round bottom flask. MPTS (2 mL) was dissolved in toluene (4 mL) and added slowly under vigorous stirring condition. The resulting mixture was stirred at 80 ℃ for 8 h. The mixture was then filtered and washed with toluene (4 mL) and double distilled water (5 mL) before drying at 100 ℃. The oxidation was carried out by contacting the sample (1.0 g) with a solution of hydrogen peroxide (33 wt%) at room temperature and stirred for 12 h. The solid was then filtered, washed abundantly with distilled water, following by drying at 100 ℃ for overnight.
2.3. General procedure for the synthesis of quinoxalinesA mixture of aromatic o-diamine (1 mmol), 1, 2-dicarbonyl compounds or phenacyl bromides (1 mmol) and NZ-PSA (0.01 g) in 5 mL of water was stirred at room temperature for an appropriate time (Scheme 2). The progress of the reaction was monitored by TLC. After completion of the reaction, the catalyst was filtered off. The solvent was evaporated under reduced pressure and the pure product was obtained without any further purification and their spectroscopic data are shown in supporting information.
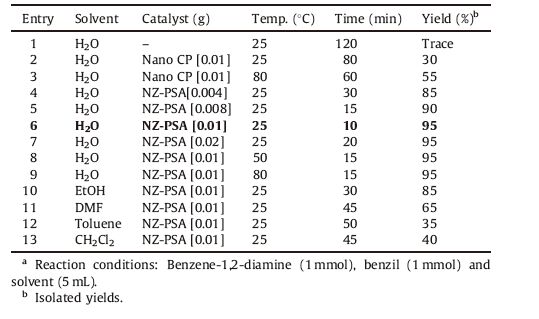
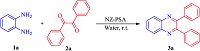
3. Results and discussionThe nanozeolite CP was prepared according to a simple method developed recently by our group [18a] and subsequently activated with 4 mol/L sulfuric acid. The activated nanozeolite CP was designated as AT-Nano CP. AT-Nano CP was reacted with MPTS in toluene at 80 ℃ to afforded propylsulfonic acid functionalized nanozeolite CP (NZ-PSA). Afterwards, the NZ-PSA was characterized by various techniques such as CHN, XRD, FT-IR, BET, TGA/DTA, SEM, TEM and TEM-EDS (Supporting information). After synthesis and characterizations of NZ-PSA, the catalytic activities of these nanocatalyst were explored for the synthesis of quinoxaline derivatives. In order to determine the optimum reaction conditions, the reaction of benzene-1, 2-diamine 1a (1 mmol) with benzil 2a (1 mmol) was examined as a model reaction in water at room temperature (Scheme 1).
|
Download:
|
| Scheme 1.The model reaction for the synthesis of quinoxaline 3a | |
The model reaction was carried out in the presence of different catalytic amounts of nanozeolite CP and NZ-PSA the results are presented in Table 1. In the absence of catalyst, only a trace amount of desired product was obtained even after in longer reaction time (entry 1). The results show that both nanocatalysts could promote the reaction, but NZ-PSA catalyst is significantly more effective than nanozeolite CP in the synthesis of quinoxaline 3a and it provides better results with high yields and short reaction times. In order to optimize the reaction conditions, the catalytic efficiency was studied with various amounts of nano NZ-PSA in the model reaction (entries 4-6). The results reveal that 0.01 g of NZ-PSA provided the best effects in terms of economy of catalyst charge and purity of products (entry 6). Moreover, higher amounts of the catalyst (0.02 g) did not improve the yield and the reaction time (entry 7). The role of solvents in the reaction was also screened. As shown in Table 1, entries 10-13, it was found that water is a suitable solvent to produce the target products in high to excellent yields and relatively short reaction time in comparison with other solvents. Also, we carried out the model reaction at various temperatures ranging from 25 ℃ to 80 ℃ (entries 7-9). The results demonstrate that increase in the reaction temperature did not affect the product yield and reaction time. Consequently, the best results were afforded by the reaction of these components in water (5 mL) in the presence of 0.01 g of NZ-PSA at room temperature obtaining quinoxaline 3a in a 95% yield (entry 6).
|
|
Table 1 Optimization of reaction conditions for the synthesis of quinoxaline 3aa. |
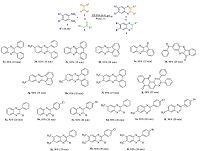
To assess the generality of this approach for the synthesis of quinoxalines, various substituted 1, 2-diketones were reacted with structurally and electronically diverse o-phenylenediamines, and the results are summarized in Scheme 2, 3a-j. It was observed that electron-donating groups had no significant effect on the reaction results (3b, g, h, i). Moreover, other 1, 2-diketones such as 9, 10- phenanthraquinone (3c, h, i), acenaphthoquinone (3d, g), and indantrione (3e, f) were examined in this reaction and corresponding quinoxalines were produced in the short time and excellent yield. In following to further explore the potential of this protocol, we also examined the synthesis of quinoxalines using another reactant, phenacyl bromide derivatives instead of 1, 2-diketones under similar reaction conditions (Scheme 2). Results demonstrate that all p-chloro and bromophenacylbromide were reacted with ophenylenediamines to provide the corresponding products in good yields (5a-i).
|
Download:
|
| Scheme 2.Synthesis of quinoxalines 3 and 5 catalyzed by NZ-PSA. Reaction conditions: o-Phenylenediamines (1 mmol), 1, 2-diketones or phenacyl bromides (1 mmol), water (5 mL) and NZ-PSA (0.01 g), 25 ℃. | |
The reaction was clean and the products were obtained in high yields without the formation of any by-products. All the products prepared were known compounds and their structures were characterized with use of the spectral methods (1H NMR and 13C NMR) and comparison with authentic samples (Supporting information). The recyclability of the catalyst for reactions was investigated for the synthesis of quinoxaline under the optimized reaction conditions. The catalyst was recovered by filtration technique after each experiment and washed with hot distilled ethanol (2 mL) twice and drying at 80 ℃ in an oven to provide an opportunity for recycling experiments. The separated nanocatalyst was reused successively eight times without any significant loss of activity (Fig. S7 in Supporting information). The strong interaction of MPTS grafted on the surface of AT-Nano CP could be the reason for the repetitive use of the catalyst in a greater number of catalytic runs with high yield.
4. ConclusionIn summary, propylsulfonic acid functionalized nanozeolite CP was easily prepared, fully characterized and used as a highly efficient heterogeneous catalyst for the synthesis of quinoxaline derivatives in water. The spectroscopic analyses of nanocatalyst indicate that the propylsulfonic acid attached on the surface of zeolite. It is observed that propylsulfonic acid functionalized nanozeolite CP gives the highest catalytic activity compared to that of nanozeolite CP. This new nanocatalyst is thermally stable, inexpensive, and easy to prepare. In addition, it could be easily separated from the reaction mixture and reused for several times without any significant loss of its activity.
AcknowledgmentThis research is supported by the Islamic Azad University, Ayatollah Amoli Branch, I. R. Iran.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2015. 04.037.
| [1] | A. Burguete, E. Pontiki, D. Hadjipavlou-Litina, Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1, 4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4, 5-dihydro-(1H)-pyrazole analogues, Bioorg. Med. Chem. Lett. 17(2007) 6439-6443. |
| [2] | J.Y. Jaung, Synthesis and halochromism of new quinoxaline fluorescent dyes, Dyes Pigm. 71(2006) 245-250. |
| [3] | Y.L. Chen, K.C. Fang, J.Y. Sheu, S.L. Hsu, C.C. Tzeng, Synthesis and antibacterial evaluation of certain quinolone derivatives, J. Med. Chem. 44(2001) 2374-2377. |
| [4] | D.J. Brown, Quinoxalines:supplement II, in:E.C. Taylor, P. Wipf (Eds.), The Chemistry of Heterocyclic Compounds, vol. 61, John Wiley & Sons, New Jersey, 2004. |
| [5] | S.V. More, M.N.V. Sastry, C.F. Yao, Cerium (IV) ammonium nitrate (CAN) as a catalyst in tap water:a simple, proficient and green approach for the synthesis of quinoxalines, Green Chem. 8(2006) 91-95. |
| [6] | H.R. Darabi, S. Mohandessi, K. Aghapoor, F. Mohsenzadeh, A recyclable and highly effective sulfamic acid/MeOH catalytic system for the synthesis of quinoxalines at room temperature, Catal. Commun. 8(2007) 389-392. |
| [7] | A. Hasaninejad, A. Zare, M.A. Zolfigol, M. Shekouhy, Zirconium tetrakis(dodecyl sulfate)[Zr(DS)4] as an efficient lewis acid-surfactant combined catalyst for the synthesis of quinoxaline derivatives in aqueous media, Synth. Commun. 39(2009) 569-579. |
| [8] | A. Dhakshinamoorthy, K. Kanagaraj, K. Pitchumani, Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature, Tetrahedron Lett. 52(2011) 69-73. |
| [9] | C.R. Qi, H.F. Jiang, L.B. Huang, Z.W. Chen, H.J. Chen, DABCO-catalyzed oxidation of deoxybenzoins to benzils with air and one-pot synthesis of quinoxalines, Synthesis (2011) 387-396. |
| [10] | X.Z. Zhang, J.X. Wang, L. Bai, Microwave-assisted synthesis of quinoxalines in PEG-400, Synth. Commun. 41(2011) 2053-2063. |
| [11] | H.M. Bachhav, S.B. Bhagat, V.N. Telvekar, Efficient protocol for the synthesis of quinoxaline, benzoxazole and benzimidazole derivatives using glycerol as green solvent, Tetrahedron Lett. 52(2011) 5697-5701. |
| [12] | C. Zhang, Z.J. Xu, L.R. Zhang, N. Jiao, Et3N-catalyzed oxidative dehydrogenative coupling of α-unsubstituted aldehydes and ketones with aryl diamines leading to quinoxalines using molecular oxygen as oxidant, Tetrahedron 68(2012) 5258-5262. |
| [13] | F. Pan, T.M. Chen, J.J. Cao, J.P. Zou, W. Zhang, Ga(ClO4)3-catalyzed synthesis of quinoxalines by cycloaddition of α-hydroxyketones and o-phenylenediamines, Tetrahedron Lett. 53(2012) 2508-2510. |
| [14] | A. Kumbhar, S. Kamble, M. Barge, G. Rashinkar, R. Salunkhe, Brönsted acid hydrotrope combined catalyst for environmentally benign synthesis of quinoxalines and pyrido[2, 3-b] pyrazines in aqueous medium, Tetrahedron Lett. 53(2012) 2756-2760. |
| [15] | A.V. Narsaiah, J.K. Kumar, Glycerin and CeCl3·7H2O:a new and efficient recyclable reactionmediumfor thesynthesisofquinoxalines, Synth.Commun.42(2012)883-892. |
| [16] | J.A. Rabo, Unifying principles in zeolite chemistry and catalysis, Catal. Rev.:Sci. Eng. 23(1981) 293-313. |
| [17] | H.S. Nalwa, Handbook of Surfaces and Interfaces of Materials, Academic Press, New York, 2001. |
| [18] | S.M. Baghbanian, N. Rezaei, H. Tashakkorian, Nanozeolite clinoptilolite as a highly efficient heterogeneous catalyst for the synthesis of various 2-amino-4 H-chromene derivatives in aqueous media, Green Chem. 15(2013) 3446-3458. |
 2015, Vol.26
2015, Vol.26