Nitrogen-containing heterocyclic compounds have been widely used in pharmaceuticals,and designing novel heterocyclic motifs has become an increasingly urgent mission for chemists and biologists [1]. Among them, 1,6-naphthyridine and their fused analogs are an important pharmacophore present in many natural [2] and designed synthetic products of therapeutic value. They are associated with a wide spectrum of biological effects such as anticancer [3], anti-HIV-1 [4], antimicrobial [5] and cytotoxic activities [6]. Therefore, the synthesis of 1,6-naphthyridine derivatives has aroused great interest in organic and medicinal chemistry communities [7, 8, 9, 10, 11].
In addition, thienopyridines [12] and their annulated with heterocycles [13] have attracted widespread interest owing to their presence in natural products,and their biological and pharmacological activities.
The Pictet-Spengler reaction [14] has become one of the most prominent strategies for carbon-carbon bond formation in synthetic organic chemistry with excellent functional group tolerance, regio- and stereo-selectivity. From this perspective, the modified Pictet-Spengler reactions are attained considerable important for the synthesis of various products and novel heterocycles of biological interest [15].
In our previous studies [16] we reported the synthesis of fused nitrogen-containing ring systems,in this paper the application of Pictet-Spengler reaction is described for the synthesis of novel benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6]naphthyridine ring systems (Scheme 1).
|
Download:
|
| Scheme 1.Syntheses of benzo[b]pyridino[30,20:4,5]thieno[2,3-e][1,6]naphthyridine-8-ones. | |
2.1. Preparation of 4-(3-aminothieno[2,3-b]pyridin-2-yl)quinoline- 2-ones (3)
To a solution of 4-bromomethylquinoline-2-one 1 [17] (20.0 mmol) in DMF (25 mL) was added 3-cyanopyridine-2-thione 2 (4.11 g, 30.0 mmol) and anhydrous potassium carbonate (5.52 g, 40.0 mmol). The mixture was heated at 80 ℃ for 6 h. After cooling to room temperature, water (50 mL) was added and stirred for 20 min. The solid was filtered and recrystallized from HOAc to give 3.
3a: 78%. Mp > 300 ℃. IR (KBr, cm-1): ν 3441,3360 (NH2), 1687 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.80 (s,3H), 6.94 (s,1H), 7.28-7.30 (m,1H), 7.39-7.42 (m,1H), 7.7 (d, 1H, J = 8.4 Hz), 7.63- 7.67 (m,1H), 7.85 (d, 1H, J = 9.3 Hz), 8.00 (d, 1H, J = 9.3 Hz), 8.64- 8.65 (m, 1H). 13C NMR (100 MHz, CF3CO2D): δ 29.6, 108.7, 114.7, 119.2,119.6, 122.5, 124.1,127.3, 127.7, 128.6, 131.4,133.9, 140.3, 142.4,147.1,159.8, 161.5. Anal. Calcd. for C17H13N3OS: C 66.43, H 4.26, N 13.67, S 10.43. Found: C 66.57, H 4.38, N 13.79, S 10.52.
3b: 83%. Mp > 300 ℃. IR (KBr, cm-1): ν 3417, 3373 (NH2), 1695 (C=O). 1H NMR (400 MHz, CF3CO2D): δ 3.85 (s,3H), 4.27 (s,3H), 7.32 (s,1H), 7.71 (s,1H), 7.75 (d, 1H, J = 7.6 Hz), 8.06 (d,1H, J = 9.6 Hz), 8.15-8.21 (m, 1H), 8.99-9.09 (m, 2H). 13C NMR (100 MHz, CF3CO2D) δ 32.4,55.5, 106, 7, 111.0, 113.6, 114.1,119.0, 123.6, 124.4,124.7, 132.7, 140.0, 147.7, 149.0, 149.5, 158.5, 158.8, 159.7. Anal. Calcd. for C18H15N3O2S: C 64.08, H4.48, N 12.45, S 9.50. Found: C 64.16, H 4.57, N 12.59, S 9.64.
2.2. Preparation of benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6] naphthyridine-8-one derivativesA mixture of 4-(3-aminothieno[2,3-b]pyridin-2-yl)quinoline- 2-one 3 (1.0 mmol), aromatic aldehyde 4 (1.0 mmol) and p-toluenesulfonic acid (p-TsOH) (19 mg, 0.1 mmol) in DMF (20 mL) was heated for 7-12 h at 100 ℃. After the completion of the reaction judged by TLC analysis,the reaction mixture was cooled to room temperature. Water (40 mL) was added and the mixture was stirred for 30 min. The solid was filtered and recrystallized from DMF to afford the corresponding products (5a-l) [18].
3. Results and discussionThe authors demonstrated that the benzo[b]pyrido[30,20:4,5] thieno[2,3-e][1,6]naphthyridine-8-ones can be readily synthesized from 4-bromomethylquinoline-2-ones by treatment with 3- cyanopyridine-2-thione, followed by the coupling/cyclization of the intermediate amine with the corresponding aldehydes under the Pictet-Spengler conditions (Scheme 1).
In this study, the key intermediate amine 4-(3-aminothieno[2,3-b]pyridin-2-yl)quinoline-2-ones 3 was obtained by the condensation of 4-bromomethylquinoline-2-ones 1 with 3- cyanopyridine-2-thione 2 via a Thorpe-Ziegler isomerization [19] in good yield. Its structure was determined from the spectral data as well as elemental analysis.
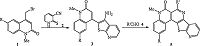
In an initial endeavor, we selected benzaldehyde 4a as a model aldehyde to react with an equimolar of the intermediate amine 3a for the preparation of benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6] naphthyridine-8-one 5a and investigated the optimal reaction conditions. The effects of solvents,catalysts and temperature were evaluatedfor this reaction, andthe results are summarizedinTable 1.
|
|
Table 1 Optimization of reaction conditions on the synthesis of benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6]naphthyridine-8-one 5a.a |
It was found that the reaction could not proceed in DMF under catalyst-free conditions (Table 1,entry 1). When the reaction was conducted using the traditional Pictet-Spengler protocol [14], in the presence of p-TsOH in DMF, the targeted compound benzo[b]pyrido [30,20:4,5]thieno[2,3-e][1,6]naphthyridine-8-one 5a was obtained in 46% yield (Table 1,entry 2). To improve the yield, different solvents and reaction temperature were evaluated. The results revealed that DMF provided much better results than toluene, CH3CN, and HOAc (Table 1,entries 2-9). Several other acids were then evaluated for their catalytic efficiency in this reaction. The results indicated that p-TsOH provided a superior catalytic effect to TFA, H2SO4 and PPA (Table 1,entries 10-13). So, the best result with a complete conversion was obtained only when the reaction was carried out in 10% p-TsOH in DMF at 100 ℃ (Table 1,entry 4).
Under the optimized conditions,a wide range of aromatic aldehydes 4 underwent this one-pot condensation with of 4-(3- amino-benzofuran-2-yl)quinoline-2-ones 3 to give the corresponding benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6]naphthyridine- 8-ones 5.
Encouraged by the above results,we investigated the scope of this reaction using various aromatic aldehydes (Table 2). A variety of electron-rich (entries 2-4 and 8-10) and electron-deficient aromatic aldehydes (entries 5, 6, 11 and 12) are effectively transformed to the corresponding naphthyridines in the presence of p-TsOH in good yields (77%-85%).
|
|
Table 2 Synthesis of benzo[b]pyrido[30,20:4,5]thieno[2,3-e][1,6]naphthyridine-8-one 5. |
All the products were characterized by IR, 1HNMR, 13CNMRand elemental analysis. And all the data are consistent with the proposed structures.
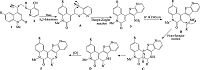
A proposed mechanism of the process is summarized in Scheme 2. The present synthetic sequence was initiated by an S-alkylation of 4-bromomethylquinoline-2-ones 1 with 3-cyanopyridine-2- thione 2 giving rise to the thioethers A. An intramolecular carbanion addition across the nitrile occurred via a Thorpe-Ziegler isomerization reaction, resulting in the formation of 4-(3- aminothieno[2,3-b]pyridin-2-yl)quinoline-2-ones 3. Next, substrates 3 underwent a cationic π-cyclization [20] with aldehydes as one-carbon electrophiles in imine intermediates B under Pictet- Spengler cyclization conditions,which resulted in the closure at the C3 position of quinoline-2-ones to give pentacyclic benzo[b]- pyrido[30,20:4,5]thieno[2,3-e][1,6]naphthyridine-8-ones ring system 5.
|
Download:
|
| Scheme 2.A proposed mechanism for the formation of 5. | |
In summary, we have developed an efficient method for the synthesis of pharmacologically important, functionalized 1,6- naphthyridine derivatives in two steps. The synthetic approach involves a Thorpe-Ziegler isomerization of 4-bromomethylquinoline- 2-ones followed by a Pictet-Spengler cyclization of the corresponding 4-(3-aminobenzofuran-2-yl)quinoline-2-ones with aromatic aldehydes in the presence of p-TsOH. This method has the advantages of using utilizes easily accessible starting materials,mild reaction conditions,straightforward product isolation and good yields.
| [1] | (a) J. Clardy, C. Walsh, Lessons from nature molecules, Nature 432(2004) 829-837;(b) J. Kato, Y. Ito, R. Ijuin, et al., Novel strategy for synthesis of substituted benzimidazo[1,2-a]quinolines, Org. Lett. 15(2013) 3794-3797;(c) L.Z. Gao, Y.S. Xie, T. Li, et al., Synthesis and antibacterial activity of novel[1,2,4] triazolo[3, 4-h] [1,8] naphthyridine-7-carboxylic acid derivatives, Chin. Chem. Lett. 26(2015) 149-151. |
| [2] | H. Nakamura, J. Kobayashi, Y. Ohizumi, Isolation and structure of aaptamine a novel heteroaromatic substance possessing a-blocking activity from the sea sponge Aaptos, Tetrahedron Lett. 23(1982) 5555-5558. |
| [3] | J.J. Bowling, H.K. Pennaka, K. Ivey, et al., Antiviral and anticancer optimization studies of the DNA-binding marine natural product aaptamine, Chem. Biol. Drug Des. 71(2008) 205-215. |
| [4] | W. Gul, N.L. Hammond, M. Yousaf, et al., Modification at the C9 position of the marine natural product isoaaptamine and the impact on HIV-1, mycobacterial, and tumor cell activity, Bioorg. Med. Chem. 14(2006) 8495-8505. |
| [5] | G.R. Pettit, H. Hoffmann, J. McNulty, et al., Antineoplastic agents 380. Isolation and X-ray crystal structure determination of isoaaptamine from the Republic of Singapore Hymeniacidon sp. and conversion to the phosphate prodrug hystatin 1, J. Nat. Prod. 67(2004) 506-509. |
| [6] | L.W. Deady, M.L. Rogers, L. Zhuang, et al., Synthesis and cytotoxic activity of carboxamide derivatives of benzo[b] [1,6] naphthyridin-(5H)ones, Bioorg. Med. Chem. 13(2005) 1341-1355. |
| [7] | (a) E.L. Larghi, M.L. Bohn, T.S. Kaufman, Aaptamine and related products. Their isolation, chemical syntheses, and biological activity, Tetrahedron 65(2009) 4257-4282;(b) Y. Takahashi, T. Kubota, A. Shibazaki, et al., Nakijinamines C-E, new heteroaromatic alkaloids from the sponge Suberites species, Org. Lett. 13(2011) 3016-3019;(c) L. Caixia, T. Xuli, L. Pinglin, et al., Suberitine A-D, four new cytotoxic dimeric aaptamine alkaloids from the marine sponge Aaptos suberitoides, Org. Lett. 14(2012) 1994-1997. |
| [8] | C. Mukhopadhyay, P. Das, R.J. Butcher, An expeditious and efficient synthesis of highly functionalized[1,6] naphthyridines under catalyst-free conditions in aqueous medium, Org. Lett. 13(2011) 4664-4667. |
| [9] | P.W. Phuan, M.C. Kozlowski, Convenient preparation of naphthyridines from halopyridines:sequential Heck coupling and cyclization, Tetrahedron Lett. 42(2001) 3963-3965. |
| [10] | (a) A. Chandra, B. Singh, S. Upadhyay, et al., Copper-free Sonogashira coupling of 2-chloroquinolines with phenyl acetylene and quick annulation to benzo[b] [1,6]-naphthyridine derivatives in aqueous ammonia, Tetrahedron 64(2008) 11680-11685;(b) R.M. Singh, R. Kumar, N. Sharma, et al., Palladium-catalyzed one-pot synthesis of benzo[b] [1,6] naphthyridines via Sonogashira coupling and annulation reactions from 2-chloroquinoline-3-carbonitriles, Tetrahedron 69(2013) 9443-9450. |
| [11] | M. Piltan, I. Yavari, L. Moradi, Tandem synthesis of functionalized hexaalkyl benzoisoquinolinopyrrolonaphthyridine-hexacarboxylate, via isoquinoline based multi-component reaction, Chin. Chem. Lett. 24(2013) 979-983. |
| [12] | V.P. Litvinov, V.V. Dotsenko, S.G. Krivokolysko, The chemistry of thienopyridine, Adv. Heterocycl. Chem. 93(2007) 117-178. |
| [13] | (a) C.G. Dave, P.R. Shah, A.B. Shah, K.C. Dave, et al., Synthesis and biological activity of pyrido[3', 2':4,5] thieno[3, 2-d]pyrimidines, J. Indian Chem. Soc. 66(1989) 48-50;(b) V.L. Ivanov, V.A. Artemov, L.A. Rodinovskaya, et al., New approaches to the synthesis of functionally substituted pyrido[3', 2':4,5] thieno[3, 2-b]pyridines and the structure of the product obtained, Chem. Heterocycl. Compd. 32(1996) 105-111;(c) J.M. Quintela, C. Peinador, C. Veiga, et al., Synthesis and antiallergic activity of pyridothienopyrimidines, Bioorg. Med. Chem. Lett. 6(1998) 1911-1925;(d) L.A. Rodinovskaya, A.M. Shestopalov, A.V. Gromova, et al., One-pot synthesis of diverse 4-di(tri)fluoromethyl-3-cyanopyri dine-2(1H)-thiones and their utilities in the cascade synthesis of annulated heterocycles, J.Comb.Chem.10(2008) 313-322;(e) A.K. Elansary, A.A.Moneer, H.H. Kadry, et al., Synthesis and anticancer activity of some novel fused pyridine ring system, Arch. Pharm. Res. 35(2012) 1909-1917. |
| [14] | A. Pictet, T.T. Spengler, Über die bildung von isochinolin-derivaten durch einwirkung von methylal auf phenyl-thylamin, phenyl-alanin und tyrosin, Ber. Dtsch. Chem. Ges. 44(1911) 2030-2036. |
| [15] | (a) S.W. Youn, The Pictet-Spengler reaction:efficient carbon-carbon bond forming reaction in heterocyclic synthesis, Org. Prep. Proced. Int. 38(2006) 505-591;(b) B. Kundu, P.K. Agarwal, S.K. Sharma, et al., Pictet-Spengler reaction revisited:engineering of tetherd biheterocycles into annulated polyheterocycles, Curr. Org. Synth. 9(2012) 357-376. |
| [16] | (a) D.L. Wang, S.F. Li, W. Li, et al., An efficient synthesis of 3-(guaiazulene-1-yl)succinimides by addition of guaiazulene to maleimides, Chin. Chem. Lett. 22(2011) 789-792;(b) D.L. Wang, Q.T. Cui, S.S. Feng, et al., A new synthesis approach to azuleno[2,1-b]pyridine-4(1H)-ones, Heterocyles 85(2012) 697-704;(c) D.L. Wang, Z. Dong, Q.T. Cui, et al., Synthesis of some pyrazole-fused pyrido[3, 2-a]azulenes, Heterocycles 87(2013) 2343-2350;(d) D.L. Wang, Z. Dong, Z. Liu, et al., Efficient one-pot synthesis of 1,4-dihydropyridino[3, 2-c]coumarins, Chin. J. Org. Chem. 34(2014) 783-787;(e) D.L. Wang, J.Y. Wu, D. Wu, et al., An efficient synthesis of 1-oxo-1,2-dihydrobenzo[b] [1,6] naphthyridine-4-carbonitriles, Chin. Chem. Lett. 25(2014) 1555-1558;(f) D.L. Wang, D. Wu, W. Zhao, et al., An efficient synthesis of benzo[b]benzofurano[2,3-e]-[1,6] naphthyridine-8-ones, Chin. Chem. Lett. 26(2015) 251-254. |
| [17] | (a) D.J. Cook, R.E. Bowen, E. Daniels, Bromination studies of alkyl-substituted 2-pyridones and 2-quinolones, J. Org. Chem. 26(1961) 4949-4955;(b) L.J. Zhang, H. Zhang, Y.H. Yang, et al., Synthesis of diethyl ribamipide carboxylate, J. Wuhan. Inst. Tech. 31(2009) 23-25. |
| [18] | Physical and spectral (IR, NMR, Anal.) data:. 5a:Mp > 300℃. IR (KBr, cm-1):ν 1675(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.78(s, 3H), 7.55-7.62(m, 7H), 7.79-7.80(m, 2H), 8.81-8.83(m, 2H), 8.98-8.90(m, 1H). 13C NMR (100MHz, CF3CO2D):δ 30.4, 115.1, 117.5, 117.6, 120.7, 122.8, 124.6, 127.5, 127.8, 127.9, 128.1, 128.4, 131.9, 132.1, 138.5, 139.9, 143.4, 150.3, 151.1, 160.1, 160.9, 162.4. Anal. Calcd. for C24H15N3OS:C 73.26, H 3.84, N 10.68, S 8.15. Found:C 73.38, H 3.97, N 10.84, S 8.34; 5b:Mp > 300℃. IR (KBr, cm-1):ν 1685(C=O). 1H NMR (400 MHz, CF3CO2D):δ 2.45(s, 3H), 3.73(s, 3H), 7.32(d, J=7.6 Hz, 2H), 7.51(d, J=7.6 Hz, 2H), 7.52-7.56(m, 3H), 7.74(t, J=8.0 Hz, 1H), 8.80-8.82(m, 2H), 8.89(d, J=8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D):δ 29.7, 30.6, 114.5, 115.9, 117.6, 120.0, 121.5, 122.2, 123.6, 126.8, 127.2, 127.7, 128.2, 129.1, 129.4, 131.4, 131.7, 133.3, 138.6, 149.8, 151.4, 160.1, 161.1. Anal. Calcd. for C25H17N3OS:C 73.69, H 4.21, N 10.31, S 7.87. Found:C 73.86, H 4.43, N 10.40, S 7.96; 5c:Mp> 300℃. IR (KBr, cm-1):ν 1676(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.91(s, 3H), 4.01(s, 3H), 7.23(d, J=8.0 Hz, 1H), 7.33-7.36(m, 2H), 7.61(t, J=8.0 Hz, 1H), 7.86-7.92(m, 2H), 8.16(t, J=8.0Hz, 1H), 8.34-8.35(m, 1H), 8.96(d, J=9.6 Hz, 1H), 9.37-9.38(m, 1H), 9.88(d, J=8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D):δ 31.3, 54.6, 111.2, 113.2, 114.4, 115.0, 116.0, 116.8, 117.8, 118.6, 112.0, 123.7, 124.9, 126.6, 128.1, 129.4, 131.3, 133.3, 137.6, 141.4, 141.8, 145.4, 146.2, 153.8, 158.4. Anal. Calcd. for C25H17N3O2S:C 70.90, H 4.05, N 9.92, S 7.57. Found:C 71.04, H 4.14, N 10.08, S 7.64; 5d:Mp> 300℃. IR (KBr, cm-1):ν 1685(C=O). 1H NMR (400MHz, CF3CO2D):δ 4.22(s, 3H), 4.32(s, 3H), 7.51-7.53(m, 2H), 7.94-7.95(m, 2H), 8.16-8.21(m, 2H), 8.45-8.46(m, 1H), 8.67-8.68(m, 1H), 9.21-9.22(m 1H), 9.66-9.67(m, 1H), 10.21-10.22(m, 1H). 13C NMR (100MHz, CF3CO2D):δ 31.3, 55.2, 114.9, 115.3, 115.6, 117.2, 124.1, 124.9, 126.2, 127.0, 128.2, 129.1, 130.6, 137.6, 137.9, 141.7, 142.3, 146.5, 146.7, 153.9, 159.2, 159.6, 162.8. Anal. Calcd. for C25H17N3O2S:C 70.90, H 4.05, N 9.92, S 7.57. Found:C 70.98, H 4.17, N 10.05, S 7.68; 5e:Mp > 300℃. IR (KBr, cm-1):ν 1686(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.92(s, 3H), 7.57-7.62(m, 4H), 7.91-7.93(m, 2H), 8.17-8.19(m, 1H), 8.36-8.37(m, 1H), 8.97-8.99(m, 1H), 9.38-9.39(m, 1H), 9.88-9.89(m, 1H). 13C NMR (100MHz, CF3CO2D):δ 31.1, 115.2, 116.6, 118.5, 125.6, 126.6, 127.3, 128.2, 128.5, 128.8, 129.4, 129.6, 129.9, 130.0, 137.7, 141.4, 145.3, 146.2, 147.4, 153.8, 158.5, 158.8. Anal. Calcd. for C24H14ClN3OS:C 67.36, H 3.30, N 9.82, S 7.49. Found:C 67.52, H 3.46, N 9.96, S 7.58; 5f:Mp > 300℃. IR (KBr, cm-1):ν 1675(C=O). 1HNMR (400MHz, CF3CO2D):δ 3.89(s, 3H), 7.29-7.31(m, 2H), 7.62-7.64(m, 2H), 7.86-7.90(m, 2H), 8.14-8.15(m, 1H), 8.34-8.36(m, 1H), 8.93(d, J=7.6Hz, 1H), 9.35-9.36(m, 1H), 9.87(d, J=7.6 Hz, 1H). 13C NMR (100 MHz, CF3CO2D):δ 30.5, 114.7, 114.9, 115.2, 117.4, 117.6, 120.8, 122.9, 127.5, 128.0, 130.3, 130.4, 132.0, 138.7, 139.9, 150.4, 151.2, 159.8, 160.1, 161.4, 162.1, 163.9. Anal. Calcd. for C24H14FN3OS:C 70.06, H 3.43, N 10.21, S 7.79. Found:C 70.18, H 3.59, N 10.38, S 7.85; 5g:Mp> 300℃. IR (KBr, cm-1):ν 1677(C=O). 1H NMR (400MHz, CF3CO2D):δ 3.72(s, 3H), 4.08(s, 3H), 7.35-7.37(m, 1H), 7.45-7.52(m, 4H), 7.60-7.61(m, 3H), 8.32(s, 1H), 8.84-8.86(m, 1H), 8.93(d, J=7.6 Hz, 1H). 13CNMR(100MHz, CF3CO2D):δ 30.6, 55.9, 110.1, 116.4, 117.8, 118.1, 120.0, 120.7, 124.4, 128.3, 128.4, 128.6, 132.2, 134.3, 137.7, 138.3, 140.5, 150.1, 150.9, 155.2, 159.7, 161.2, 162.0. Anal. Calcd. for C25H17N3O2S:C 70.90, H 4.05, N 9.92, S 7.57. Found:C 71.04, H 4.18, N 10.05, S 7.69; 5h:Mp > 300℃. IR (KBr, cm-1):ν 1686(C=O). 1H NMR (400 MHz, CDCl3):δ 2.45(s, 3H), 3.75(s, 3H), 4.08(s, 3H), 7.33(d, J=8.0 Hz, 2H), 7.45-7.47(m, 3H), 7.52(d, J=9.2 Hz, 1H), 7.76-7.78(m, 1H), 8.26(s, 1H), 8.95-8.96(m, 1H), 9.19(d, J=7.6 Hz, 1H). 13CNMR (100MHz, CF3CO2D):δ 21.6, 30.6, 55.9, 110.0, 116.3, 117.7, 118.1, 120.0, 120.7, 124.3, 128.2, 128.4, 128.6, 132.0, 134.2, 137.7, 138.2, 140.5, 150.2, 151.0, 155.2, 159.7, 161.1, 162.2. Anal. Calcd. for C26H19N3O2S:C 71.38, H 4.38, N 9.60, S 7.33. Found:C 71.47, H 4.53, N 9.74, S 7.48; 5i:Mp > 300℃. IR (KBr, cm-1):ν 1688(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.75(s, 3H), 3.89(s, 3H), 4.06(s, 3H), 7.03-7.05(m, 2H), 7.33-7.35(m, 1H), 7.46-7.47(m, 1H), 7.59-7.61(m, 3H), 8.29(s, 1H), 8.33-8.45(m, 1H), 8.94-8.96(m, 1H). 13C NMR (100 MHz, CF3CO2D):δ 30.5, 55.3, 55.9, 110.1, 113.3, 114.0, 116.4, 117.8, 118.1, 120.0, 120.8, 121.1, 124.6, 128.1, 128.7, 132.1, 134.3, 138.1, 144.7, 150.1, 151.1, 155.2, 159.1, 159.5, 160.8, 162.1. Anal. Calcd. for C26H19N3O3S:C 68.86, H 4.22, N 9.27, S 7.07. Found:C 68.98, H 4.37, N 9.41, S 7.16; 5j:Mp > 300℃. IR (KBr, cm-1):ν 1680(C=O). 1H NMR (400MHz, CDCl3):δ 3.73(s, 3H), 3.90(s, 3H), 4.09(s, 3H), 7.49-7.55(m, 5H), 7.59-7.61(m, 2H), 7.75(t, J=8.0 Hz, 1H), 8.81-8.83(m, 2H), 8.87(d, J=8.0 Hz, 1H). 13C NMR (100 MHz, CF3CO2D):δ 30.7, 55.3, 56.0, 110.1, 113.3, 116.4, 117.6, 118.2, 120.0, 120.8, 124.2, 128.2, 130.2, 132.1, 134.2, 135.6, 138.4, 150.1, 151.0, 155.2, 159.6, 159.8, 160.7, 162.1. Anal. Calcd. for C26H19N3O3S:C 68.86, H 4.22, N 9.27, S 7.07. Found:C 69.02, H 4.35, N 9.43, S 7.19; 5k:Mp > 300℃. IR (KBr, cm-1):ν 1677(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.74(s, 3H), 4.07(s, 3H), 7.37(d, J=8.8 Hz, 1H), 7.47-7.49(m, 3H), 7.55-7.57(m, 3H), 8.30(s, 1H), 8.85-8.86(m, 1H), 8.90-8.92(m, 1H). 13CNMR(100 MHz, CF3CO2D):δ 30.6, 56.0, 110.2, 116.5, 117.3, 118.1, 120.0, 120.9, 124.9, 128.0, 128.2, 129.9, 132.3, 134.0, 134.2, 138.4, 141.7, 151.1, 150.9, 155.4, 159.6, 159.9, 161.9. Anal. Calcd. for C25H16ClN3O2S:C 65.57, H 3.52, N 9.18, S 7.00. Found:C 65.72, H 3.64, N 9.31, S 7.13; 5l:Mp > 300℃. IR (KBr, cm-1):ν 1685(C=O). 1H NMR (400 MHz, CF3CO2D):δ 3.74(s, 3H), 4.06(s, 3H), 7.20(m, 2H), 7.37-7.39(m, 1H), 7.47-7.49(m, 1H), 8.60-8.62(m, 3H), 8.30(s, 1H), 8.85-8.86(m, 1H), 7.96-7.98(m, 1H). 13C NMR (100 MHz, CF3CO2D):δ 30.6, 56.0, 110.1, 114.7, 114.9, 116.5, 118.1, 120.2, 120.9, 124.7, 128.1, 130.3, 130.4, 132.1, 134.2, 136.9, 138.4, 151.2, 155.3, 156.3, 159.7, 159.9, 162.2. Anal. Calcd. for C25H16FN3O2S:C 68.01, H 3.65, N 9.52, S 7.26. Found:C 68.15, H 3.78, N 9.69, S 7.42. |
| [19] | (a) A.M. Shestopalov, A.E. Fedorov, P.A. Belyakov, Study of the orientation of the Thorpe-Ziegler reaction, Chem. Heterocycl. Comp. 36(2000) 609-610;(b) V. Gefenas,Ž. Stankevičūte, A. Malinauskas, Novel method for the synthesis of furo[2,3-d]pyrimidines by cyclization of 4-(phenacyloxy)pyrimidine-5-carbonitriles, Chem. Heterocycl. Comp. 46(2010) 372-374;(c) H.F. Zhang, Z.Q. Ye, G. Zhao, Enantioselective synthesis of functionalized fluorinated dihydropyrano[2,3-c]pyrazoles catalyzed by a simple bifunctional diaminocyclohexane-thiourea, Chin. Chem. Lett. 25(2014) 535-540. |
| [20] | P.K. Agarwal, M. Saifuddin, B. Kundu, Regioselective intramolecular electrophilic substitution reactions involving π-deficient pyridine substrates:a new entry to pyridoquinazolines and benzo[h] [1,6] naphthyridines, Tetrahedron 66(2010) 862-870. |
 2015, Vol.26
2015, Vol.26