b Engineering Research Center of Materials-Oriented Chemical Engineering of Xinjiang Production and Construction Corps, Shihezi 832003, China;
c Key Laboratory of Materials-Oriented Chemical Engineering of Xinjiang Uygur Autonomous Region, Shihezi 832003, China
Vinyl chloridemonomer (VCM) ismainly used in the synthesis of polyvinyl chloride (PVC), which is one of the world’s five major engineeringplastics andwidelyusedinallwalks of life. The acetylene hydrochlorination reactionwas the earliest process for the industrial production of vinyl chloride [1, 2]. Up to date, China has been the world’s largest PVCproductioncountry, whichproduces20, 000, 000 t of PVC annually, accounting for 41% of total world production capacity. It isworth noting thatmore than 70% of the PVC is prepared by the acetylene hydrochlorination method in China [3, 4].
Catalyst supports have very important effects on the performance of catalysts and strongly affect the catalytic activity for the acetylene hydrochlorination. Generally, carbon-supported catalysts (especially for the HgCl2-supported activated carbon (AC), i.e., HgCl2/AC) are employed for the acetylene hydrochlorination reaction [5]. The AC as a catalyst support possesses high specific surface area, high electrical conductivity and thermal conductivity, which enhances the active components on the surface of AC and gives good catalytic efficiency [6]. However, AC is easily crushed under reaction conditions, resulting in loss of the active components HgCl2 and eventual deactivation of the catalyst. HgCl2 erosion can be toxic and a source of pollution [3, 7]. In addition, the AC-supported catalysts are difficult toregenerate owing to theirlow mechanical strength and pulverization [8]. These liabilities, along with the essential demands to incorporate special features, lead scientists toward: (a) low-cost and environmentally friendly mercury-free catalysts; (b) advanced catalyst supports with high mechanical strength and adsorption ability, which reduce the loss of mercury and enhance the acetylene hydrochlorination reaction [9, 10, 11].
Herein, we employed expanded multilayered vermiculite (EMLVMT) as a catalyst support for acetylene hydrochlorination. VMT is a natural clay mineral (aluminosilicate) and possesses a layered structure, high mechanical strength, good thermal stability and low cost [12]. Moreover, the vermiculite has considerable adsorption capacity to mercury [13]. Recently, EML-VMT has been used as catalyst supports, such as in the modified-VMT-supported Ni catalysts for CO2 methanation to syngas (SG) [14], VMTsupported TiO2 as efficient photocatalysts for water decontamination [15] and methylene blue photocatalytic degradations [16]. To date, vermiculite as a catalyst support in the acetylene hydrochlorination has not been reported. We have successfully prepared EML-VMT and employed EML-VMT as a catalyst support for the acetylene hydrochlorination. The as-obtained HgCl2/EML-VMT and HgCl2/EML-VMT-C achieved good acetylene conversions of 90.6% and 97.3%, respectively. The selectivity of vinyl chloride is 93.7% and 100%, respectively, corresponding to turnover frequency (TOF) values of 7.03 × 10-3 s-1 and 8.83 × 10-3 s-1, respectively at a temperature of 140 °C, an acetylene gas hourly space velocity of 108 h-1, and a feed volume ratio V(HCl)/V(C2H2) of 1.15.
2. Experimental 2.1. Pretreatment of raw VMTRaw VMT was purchased from Xinjiang Yuli Xinlong Vermiculite Co., Ltd., Xinjiang, China. The VMT is composed of 38.76% SiO2, 24.05% MgO, 20.04% Al2O3, 6.22% K2O, 4.93% Fe2O3, 3.03% CaO, 2.02% Na2O, 0.94% TiO2. The EML-VMT was prepared by adding VMT (5 g) into 50 mL of 25% H2O2 aqueous solution with heating in a water bath (80 °C) for 2 h. After the treatment, the hydrated VMC was dried quickly by microwave radiation (700Wfor 1 min). The dried EML-VMT was ground and sieved to a size fraction of 0.25-0.42 mm (passed through 40-60 sieve).
In order to obtain carbon-mixing EML-VMT (EML-VMT-C), the EML-VMT (2 g) was transferred to a clear and dense solution of pure glucose (22.4 g) in deionized water (80 mL). The mixture was placed in a 100 mL Teflon-sealed autoclave and maintained at 180 °C in an oven for 3 h, since glucose is carbonized at 180 °C under hydrothermal conditions. The as-prepared product was centrifuged and washed with deionized water and ethanol five times sequentially, and then dried at 80 °C for 5 h. Finally the powder was further annealed at 850 °C for 4 h in flowing nitrogen.
2.2. Catalyst preparationIn order to achieve 10 wt% HgCl2 loading, HgCl2/EML-VMT and HgCl2/EML-VMT-C catalysts were prepared using an incipient wetness impregnation technique. A solution of HgCl2 (222 mg) in deionized water (7 mL) was added drop wise to the EML-VMT (2 g) and EML-VMT-C (2 g) respectively, while stirring for 1 h at room temperature. The samples were soaked for 12 h and dried at 105 °C for 12 h.
2.3. Catalyst characterizationThe chemical compositions of raw vermiculite were determined by X-ray fluorescence (XRF, Panalytical Axios-mAX). X-ray diffraction (XRD) patterns were determined using a Bruker D8 advanced X-ray diffractometer to evaluate the crystal structure of the as-obtained samples, using monochromatic Cu Ka radiation (l = 1.5406Å) in the range 2θ = 10°-90°. The carbon deposition on used catalysts was analyzed by a thermogravimetric analysis (TGA, Netzsch STA449F3). The samples of 10-15 mg were heated at a rate of 10 K/min from room temperature to 1223 K in an oxygen flow (50 mL/min). Scanning electron microscopy (SEM, JEOL JSM- 6490LV) and transmission electron microscope (TEM, Hitachi H-600) were performed to observe the morphologies of the catalysts. The real HgCl2 content was determined by an atomic fluorescence spectrometer (AFS-810, Titan Instruments Co., Beijing).
2.4. Catalytic performance evaluationCatalytic performance was measured in a fixed bed reactor according to our previous paper [17, 18]. At first, the reactor was purged with nitrogen to remove water and air in the reacting system. Then, hydrogen chloride gas was passed through the reactor with a flow rate of 20 mL/min to activate the catalyst (1.0 g) [19]. When the reactor was heated to 140 °C, acetylene and hydrogen chloride were fed through the heated reactor at a gas hourly space velocity (GHSV) of 108 h-1. The exit gas mixture was passed through a Dreschel bottle containing water to remove hydrogen chloride. Compositions of the product were analyzed using GC-2014C gas chromatography (Shimadzu International Trading Ltd. Company). The data were collected every 40 min (i.e., 10 min, 50 min, 90 min, etc.). Acetylene conversion (XA) and VCM selectivity (SVC) were defined by the following equations.

In the above equations, φAO was defined as the volume fraction of acetylene in the raw gas, and φA was defined as the volume fraction of remaining acetylene in the product gas. φVC was the volume fraction of vinyl chloride in product gas.
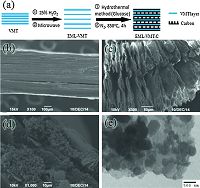
3. Results and discussionTo understand the formation mechanism of EML-VMT-C catalyst support, a schematic illustration is proposed in Fig. 1a. It mainly consists of two steps. Firstly, the raw VMT is immersed in a H2O2 aqueous solution, which can effectively ex foliate VMT and lead to EML-VMT. The excess water is then removed by microwave irradiation-assisted heat treatment, which can rapidly heat the materials [20, 21, 22]. The corresponding SEM images of the asobtained VMT and EML-VMT are shown in Fig. 1b and c. The raw VMT could be expanded more than 40 times via soaking in a H2O2 aqueous solution and the subsequent microwave treatment. Secondly, the EML-VMT-C is formed from the glucose degradation and carbonization using a hydrothermal synthesis and subsequent heat treatment at850 °C for 4 h.As shown in Fig.1 dandc, the surface of EML-VMT was coated by carbon layer and deposited by carbon nanospheres due to the glucose degradation and carbonization.
|
Download:
|
| Fig. 1.(a) Schematic illustration of the synthesis process of EML-VMT-C. SEM images of (b) VMT, (c) EML-VMT, (d) EML-VMT-C and (e) TEM image of EML-VMT-C. | |
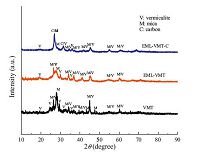
Fig. 2 shows the XRD patterns of the as-obtained products. The reflections of VMT and EML-VMT can be mainly attributed to the VMT crystal layer (PDF#74-1732) and mica (1M-phlogopite, PDF#10-0495) [23, 24, 25]. The carbon in the EML-VMT-C is detected in the XRD pattern, and the corresponding diffraction peaks appear at 2θ = 26.68 and 31.38, respectively. Moreover, a thermogravimetric analysis (TGA) confirmed the presence of carbon and gave a carbon content value of 21.8%.
|
Download:
|
| Fig. 2.The XRD patterns of VMT, EML-VMT and EML-VMT-C. | |
The HgCl2/EML-VMT and HgCl2/EML-VMT-C catalysts were evaluated under a fixed reaction condition at a temperature of 140 °C, an acetylene gas hourly space velocity (GHSV) of 108 h-1, and a feed volume ratio V(HCl)/V(C2H2) of 1.15. The real contents of HgCl2 in HgCl2/EML-VMT and HgCl2/EML-VMT-C were 9.73 wt% and 9.89 wt%, respectively. As shown in Fig. 3, theHgCl2/EML-VMT and HgCl2/EML-VMT-C exhibited initial acetylene conversions of 85.7% and 94.8% respectively, corresponding to initial VCM selectivities of 89.3% and 99.8% respectively. Unfortunately, the acetylene conversion for the HgCl2/EML-VMT started to decrease at the reaction time of 250 min. Then, the acetylene conversion showed continued slowdown and reached the value of 17.1% at 810 min. This is principally because there was much carbon deposition onto the surface of HgCl2/EML-VMT, which easily resulted in the dehydrogenation of hydrocarbons. Unlike the HgCl2/EML-VMT, the HgCl2/EML-VMT-C gave a high acetylene conversion of 97.3% at 250 min. Even after 810 min, the HgCl2/ EML-VMT-C exhibited a value of 86.7%. Moreover, the VCM selectivity for the HgCl2/EML-VMT-C catalyst was easy to reach a value of 100%, while that of the HgCl2/EML-VMT catalysed at about 92.0%. Additionally, it can be easily found that the calculated turnover frequency (TOF) values of HgCl2/EML-VMT-C catalyst were much higher than those of the HgCl2/EML-VMT catalyst (e.g., reaction time at 90, 250, 330, 410 min, respectively). Compared with the HgCl2/EML-VMT, the HgCl2/EML-VMT-C gave the highest TOF value of 8.83 × 10-3 s-1. All results indicated that the catalytic performance of the HgCl2/EML-VMT-C catalyst is much better than that of the HgCl2/EML-VMT catalyst.

|
Download:
|
| Fig. 3.(a) Acetylene conversion in acetylene hydrochlorination of HgCl2/EML-VMT and HgCl2/EML-VMT-C under a fixed reaction condition (C2H2/HCl = 1:1.15, GHSV = 108 h-1 and reaction temperature = 140 °C). (b) VCM selectivity and the corresponding TOF values (inset). | |
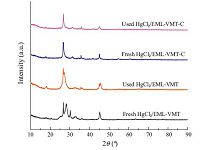
From the XRD patterns in Fig. 4, it can be seen that there are no significant variations from fresh HgCl2/EML-VMT-C to used HgCl2/EML-VMT-C. Unlike the pattern of the fresh HgCl2/EMLVMT, the used HgCl2/EML-VMT significantly changed at 26.68 due to carbon deposition in the acetylene hydrochlorination process. The amount of solid carbon deposited on the used HgCl2/EML-VMT was up to 5.48%, which indicated that hydrocarbon dehydrogenation occurred severely on the surface of HgCl2/EML-VMT in the acetylene gas flowing process [26]. It is worth noting that only 0.78% solid carbon deposited on the used HgCl2/EML-VMT-C, which strongly favored the acetylene hydrochlorination process.
|
Download:
|
| Fig. 4.XRD patterns of HgCl2/EML-VMT and HgCl2/EML-VMT-C before and after reaction. | |
4. Conclusion
A novel expanded multilayered vermiculite (EML-VMT) is successfully used as a catalyst support for the acetylene hydrochlorination. The as-obtained HgCl2/EML-VMT catalyst exhibits an initial acetylene conversion of 85.7% and the corresponding selectivity of vinyl chloride is 89.3% at a temperature of 140 °C, an acetylene gas hourly space velocity of 108 h-1, and a feed volume ratio V(HCl)/V(C2H2) of 1.15. However, the HgCl2/EMLVMT catalyst was deactivated after 300 min of use. Owing to the mixing carbon, the as-obtained HgCl2/EML-VMT-C achieved a high acetylene conversion of 97.3% and showed good stability. The corresponding selectivity of vinyl chloride was100% and the turnover frequency (TOF) value was 8.83 × 10-3 s-1. We believe that this EML-VMT represents a new way to prepare the catalysts for the acetylene hydrochlorination and can potentially be extended to the synthesis of similar superstructures.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Nos. 21163015, 21366027), the Doctor Foundation of Bingtuan (No. 2014BB004), the National Basic Research Program of China (973Program, No. 2012CB720300), the Program for Changjiang Scholars, Innovative Research Team in University (No. IRT1161), the Program of Science and Technology Innovation Team in Bingtuan (No. 2011CC001).
| [1] | X.Y. Li, M.Y. Zhu, B. Dai, AuCl3 on polypyrrole-modified carbon nanotubes as acetylene hydrochlorination catalysts, Appl. Catal. B: Environ. 142–143 (2013) 234–240. |
| [2] | J.L. Zhang, N. Liu, W. Li, B. Dai, Progress on cleaner production of vinyl chloride monomers over non-mercury catalysts, Front. Chem. Sci. Eng. 5 (2011) 514–520. |
| [3] | K. Zhou, B. Li, Q. Zhang, et al., The catalytic pathways of hydrohalogenation over metal-free nitrogen-doped carbon nanotubes, ChemSusChem 7 (2014) 723–728. |
| [4] | K. Zhou, J.C. Jia, X.G. Li, et al., Continuous vinyl chloride monomer production by acetylene hydrochlorination on Hg-free bismuth catalyst: from lab-scale catalyst characterization, catalytic evaluation to a pilot-scale trial by circulating regeneration in coupled fluidized beds, Fuel Process. Technol. 108 (2013) 12–18. |
| [5] | H. Bremer, H. Lieske, Kinetics of the hydrochlorination of acetylene on HgCl2/ active carbon catalysts, Appl. Catal. 18 (1985) 191–203. |
| [6] | C.Y. Hou, L.R. Feng, F.L. Qiu, Highly active catalyst for vinyl acetate synthesis by modified activated carbon, Chin. Chem. Lett. 20 (2009) 865–868. |
| [7] | X.Y. Li, X.L. Pan, X.H. Bao, Nitrogen doped carbon catalyzing acetylene conversion to vinyl chloride, J. Energy Chem. 23 (2014) 131–135. |
| [8] | J.H. Xu, J. Zhao, J.T. Xu, et al., Influence of surface chemistry of activated carbon on the activity of gold/activated carbon catalyst in acetylene hydrochlorination, Ind. Eng. Chem. Res. 53 (2014) 14272–14281. |
| [9] | K. Zhou, J.C. Jia, C.H. Li, et al., A low content Au-based catalyst for hydrochlorination of C2H2 and its industrial scale-up for future PVC processes, Green Chem. 17 (2015) 356–364. |
| [10] | K. Zhou, J.K. Si, J.C. Jia, et al., Reactivity enhancement of N-CNTs in green catalysis of C2H2 hydrochlorination by a Cu catalyst, RSC Adv. 4 (2014) 7766–7769. |
| [11] | X.Y. Li, X.L. Pan, L. Yu, et al., Silicon carbide-derived carbon nanocomposite as a substitute for mercury in the catalytic hydrochlorination of acetylene, Nat. Commun. 5 (2014) 3688. |
| [12] | J. Zhang, T.Y. Liu, R. Chen, X.H. Liu, Vermiculite as a natural silicate crystal for hydrogen generation from photocatalytic splitting of water under visible light, RSC Adv. 4 (2014) 406–408. |
| [13] | F.H. do Nascimento, J.C. Masini, Influence of humic acid on adsorption of Hg(II) by vermiculite, J. Environ. Manag. 143 (2014) 1–7. |
| [14] | Y.F. Liu, Z.H. He, L. Zhou, Z.S. Hou, W.M.J. Eli, Simultaneous oxidative conversion and CO2 reforming of methane to syngas over Ni/vermiculite catalysts, Catal. Commun. 42 (2013) 40–44. |
| [15] | L.C.R. Machado, C.B. Torchia, R.M. Lago, Floating photocatalysts based on TiO2 supported on high surface area exfoliated vermiculite for water decontamination, Catal. Commun. 7 (2006) 538–541. |
| [16] | Y. Sun, L. Liu, D.Z. Jia, J.H. Liu, Preparation and properties of vermiculite supported TiO2 photocatalyst, Chin. J. Inorg. Chem. 27 (2011) 40–46. |
| [17] | X.G. Wang, B. Dai, Y. Wang, F. Yu, Nitrogen-doped pitch-based spherical active carbon as a nonmetal catalyst for acetylene hydrochlorination, ChemCatChem 6 (2014) 2339–2344. |
| [18] | H.Y. Zhang, B. Dai, W. Li, et al., Non-mercury catalytic acetylene hydrochlorination over spherical activated-carbon-supported Au–Co (III)–Cu (II) catalysts, J. Catal. 316 (2014) 141–148. |
| [19] | M.Y. Zhu, L.H. Kang, Y. Su, S.Z. Zhang, B. Dai, MClx (M = Hg, Au, Ru; x = 2, 3) catalyzed hydrochlorination of acetylene-A density functional theory study, Can. J. Chem. 91 (2013) 120–125. |
| [20] | F. Yu, L.L. Zhang, M.Y. Zhu, et al., Overwhelming microwave irradiation assisted synthesis of olivine-structured LiMPO4 (M = Fe, Mn, Co and Ni) for Li-ion batteries, Nano Energy 3 (2014) 64–79. |
| [21] | F. Yu, S.H. Lim, Y.D. Zhen, Y.X. An, J.Y. Lin, Optimized electrochemical performance of three-dimensional porous LiFePO4/C microspheres via microwave irradiation assisted synthesis, J. Power Sources 271 (2014) 223–230. |
| [22] | Y.-H. Ma, G. Wu, N. Jiang, et al., Microwave-assisted, facile, rapid and solvent-free one pot two-component synthesis of some special acylals, Chin. Chem. Lett. 26 (2015) 81–84. |
| [23] | S. Ittu, N. Constantin, Some characteristics of vermiculite mineral, Metal. Int. 18 (2013) 73–76. |
| [24] | S. Hillier, E.M.M. Marwa, C.M. Rice, On the mechanism of exfoliation of ‘Vermiculite’, Clay Miner. 48 (2013) 563–582. |
| [25] | X.X. Huo, L.M. Wu, L.B. Liao, Z.G. Xia, L.J. Wang, The effect of interlayer cations on the expansion of vermiculite, Powder Technol. 224 (2012) 241–246. |
| [26] | L.L. Xu, X.G. Wang, H.Y. Zhang, et al., Application of a novel carbon carrier in acetylene hydrochlorination, Chem. Ind. Eng. Prog. 30 (2011) 536–541. |
 2015, Vol.26
2015, Vol.26 


