b Department of Food Nanotechnology, Research Institute of Food Science and Technology(RIFST), Mashhad 91735-147, Iran
A3-coupling of an aldehyde, alkyne, and amine, is considered as one of the best instances of acetylene-Mannich multicomponent reactions which has also been well recognized as a synthetic method toward propargylic amines [1,2,3,4,5]. The latter are versatile key intermediates in total synthesis of some biologically active compounds such as b-lactams, allylamines, oxotremorine analogs, isosteres, oxazoles, and other natural products, as well as therapeutics [6,7,8]. Furthermore, propargylamines are utilized as precursors in the synthesis of numerous heterocyclic compounds such as quinolines [9], phenanthrolines [10], pyrroles [10], pyrrolidines [11], indolizines [12], and oxazolidinones [7]. Classical methods for the preparation of these compounds are nucleophilic attack of lithiumacetylides or Grignard reagents on imines or their derivatives [13,14]. However, this approach involves stoichiometric amounts of reagents, requires strict control of the reaction conditions due to its moisture sensitive nature and its application is limited in the presence of the more reactive functional groups such as esters [15]. A3-coupling is an alternative atom-economical method to synthesis of propargylamines by catalytic C-H activation, where water is only the theoretical by-product [3,16]. The alkyne C-H bond can be activated by employing different complexes and salts of transition metal catalysts such as gold complexes [17], silver [18], copper [19,20,21], zinc [22], iron [23], indium [24,25], iridium [26], mercury [27], nickel [15], zirconium [28], and rhenium [29], but among them the most employed metal has been copper, especially with secondary amines [1,2,30]. Although the majority of the catalytic systems are homogeneous, their loss at the end of the reaction reduces their utility especially for industrial applications. Nowadays, metal nanoparticles are considered as heterogeneous catalysts which possess a high surface-to-volume ratio. Because of enhanced activity and selectivity, they could be competitive alternative to classical catalysis [31,32]. Particularly, the immobilization of metallic salts on high-surface-area nanoparticles causes better stability, dispersity, and recyclability of the catalyst [33]. Recently, such heterogeneous catalysts including silica-immobilized CuI [34], silica gel anchored copper chloride [35], copper ferrite nanoparticles [36], copper nanoparticles [37], nanocrystallinecopper (II) oxide [38], impregnated copper on magnetite [39], have also been employed for A3-coupling preparation of propargylamines.
On the other hand, Fe3O4 NPs is a good support for other catalysts and can facilitate their separation effectively from the liquid products of the reaction via an external magnet [40]. However it is especially difficult to obtain relatively disaggregated Fe3O4 NPs, due to its inherent magnetism. This results in losing the dispersibility and specific properties which eventually decreases its activity [41]. Therefore, immobilization of such magnetic NPs is required [42]. Two dimensional graphene (G) and its oxidized derivatives (GO) are extensively used as supports for the preparation of free-standing magnetic NPs. Unique two-dimensional lamellar structure, high surface area as well as full surface accessibility, chemical inertness and edge reactivity of GO make it a promising candidate in preventing aggregation of Fe3O4 NPs. It improves the overall catalytic activity owing to the strong synergistic interaction between GO and NPs [43].
As part of our ongoing research on the synthesis and application of Fe3O4 NPs/GO nanocomposites [44,45], here we wish to present our results on the performance and scope of Fe3O4 NPs/GO-CuO NPs as a novel nano-catalyst, in the A3-coupling preparation of propargylamines (Scheme 1).
|
Download:
|
| Fe3O4 NPs/GO–CuO NPs catalyses the A3-coupling leading to propargylamines. | |
The synthesized GO (from purified natural graphite using modified Hummer’s method [46]) is coated with Fe3O4 NPs as described in our previous report [44,45]. Briefly, 30 mg of GO in 25 mL of water was ultrasonicated for 30 min. 50 mL aqueous solution of ferric chloride (FeCl3▪6H2O, 800 mg) and ferrous chloride (FeCl3▪6H2O, 300 mg) is added at room temperature. The temperature is raised to 85 ℃ and a 30% ammonia solution is gradually added to increase the pH up to 10. After being rapidly stirred for 45 min, the solution is cooled at room temperature. Subsequently, the resulting black precipitate is centrifuged and washed three times with DI water and lastly dried at 60 ℃. For preparation of Fe3O4 NPs/GOCuO NPs nano-catalyst, 30 mg of prepared Fe3O4 NPs/GO in 100 mL of water and CuCl2▪2H2O (20 mg) is ultrasonicated for 60 min. Then 10 mL NaBH4 solution (1 wt%) is added and the solution heated in an oil bath at 100 ℃ under a water-cooled condenser for 24 h. After that, the solution was cooled at room temperature and subsequently, the resulting precipitates are centrifuged and washed three times with DI water and lastly dried at 60 ℃.
3. Results and discussion 3.1. Characterization of synthesized Fe3O4 NPs/GO-CuO NPs nanocatalystIn the FTIR (Thermo spectrometer) spectrum of the synthesized GO (Fig. 1a), the peak at 1710 cm-1 corresponds to the stretching band of C55O in carboxylic acid or carbonyl moieties. The intense band at 3418 cm-1 is attributed to stretching of the O-H and the deformation of the C-O is observed at 1167 cm-1 [47]. FTIR spectrum of Fe3O4 NPs/GO (Fig. 1b) differs from that of GO as evidenced by the disappearing of C55O bond and weakening of the O-H bond at 3415 cm-1. The peak at 1625 cm-1 (aromatic C55C) may be assigned to the skeletal vibrations of graphitic domains of GO. Also it shows the band around 558 cm-1 that is attributed to Fe-O, confirming the existence of Fe3O4 NPs [37]. FTIR spectrum of Fe3O4 NPs/GO-CuO NPs (Fig. 1c) is almost similar to Fe3O4 NPs/GO spectrum despite of a little change in the position of Fe-O bond (567 cm-1) that maybe attributed to the presence of Cu-O along with Fe-O bond in the chemical composition of nano-catalyst. SEM image (Philips XL30 microscope) indicates the fair dispersity and spherical morphology of the nanoparticles anchored on the surface of GO, with average particles sizes between 36 nm and 53 nm (Fig. 1d). The chemical composition of Fe3O4 NPs/GO-CuO NPs nano-catalyst is also examined by EDX analysis which shows peaks corresponding to C, O, Fe and Cu elements with weight percentages of 1.16%, 8.20%, 80.77 and 9.87%, respectively (Fig. 1e). In the TEM image (Philips, EM208S) of Fe3O4 NPs/GO-CuO NPs catalyst, the dark background represents graphenic substrate and black dots displays metal oxide nanoparticles distributed over the GO (Fig. 1f).

|
Download:
|
| Fig. 1.Characterization of graphene oxide (GO) supported CuO and Fe3O4 by FTIR (a-c), SEM (d) and EDX analysis (e) and TEM image (f). | |
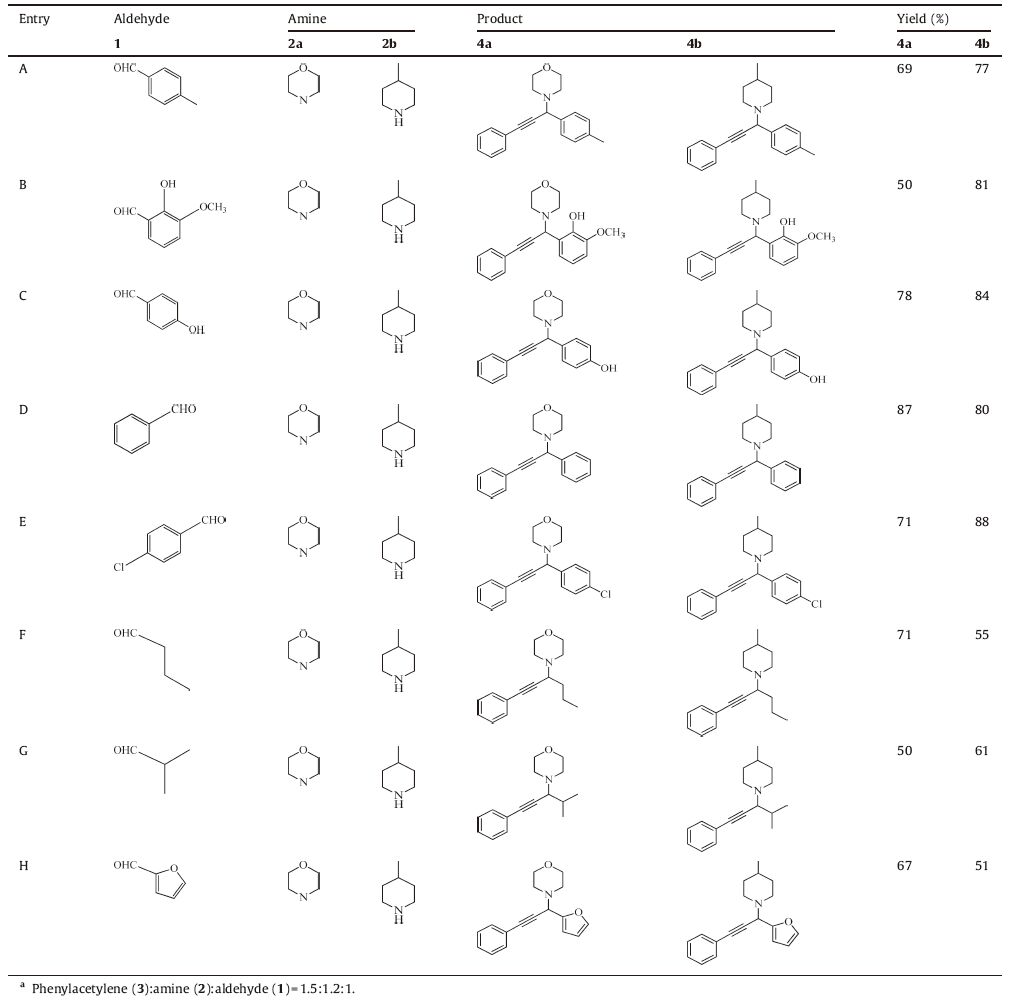
Firstly, a screening is carried out in order to optimize the temperature of the reaction, solvent and catalyst amount with the coupling of benzaldehyde (1), morpholine (2), and phenylacetylene (3) as a model reaction (Scheme 1). To check the solvent effect on the product yield, the model reaction is carried out with 20 mg of Fe3O4 NPs/GO-CuO NPs nano-catalyst in six different solvents such as toluene, MeOH, EtOH, CH2Cl2, CH3CN and DI-water for 24 h (Table 1, entries 1-6). The results show that EtOH is the most effective solvent, since the reaction proceeds smoothly giving the maximum yield (Table 1, entry 6). It is noteworthy that when water and CH2Cl2 is used as the solvent, only a trace amount of product is observed (Table 1, entries 4, 5). Employing lower amounts of nano-catalyst reduces the yield (Table 1, entries 6-9). A higher dosage of the nano-catalyst neither increases the yield nor decreases the reaction time. Thus, all the reactions are performed in EtOH with 20 mg of Fe3O4 NPs/GO-CuO NPs at 90 8C for 24 h.
|
|
Table 1 Optimization of A3-coupling the reaction conditions for synthesis of propargylamines. a |
Once the efficiency of nano-catalyst is established, using the optimized reaction conditions, the scope of the reaction is probed. Excellent results are encountered for the different combinations of aldehydes, amines, and alkynes. To generalize the applicability of our synthesized nano-catalyst in promoting the A3-coupling reaction, a variety of structurally divergent aldehydes and amines are used (Table 2).
|
|
Table 2 A3-coupling of aldehydes (1), amines (2), and alkynes (3) catalyzed by Fe3O4 NPs/GO-CuO NPs nano-catalyst for production of propargylamines (4)a. |
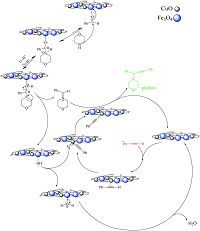
The results indicate that the aromatic aldehydes with both electron-donating and electron-withdrawing substituents display high reactivity with two different amines (2a and 2b) and generate the corresponding products (4a and 4b) in good yields (Table 2, entries A-E). Aliphatic aldehydes are also able to undergo addition to afford the corresponding 4 effectively. However, the reaction is found to be affected by the nature of 1 as aromatic aldehyde show higher reactivity, giving higher yields under the same conditions (Table 2, entries F, G). Similarly, heteroaromatic aldehyde also participated well in this protocol (Table 2, entry H). A tentative mechanism for the catalyzed A3-coupling over Fe3O4 NPs/GO-CuO NPs is proposed involving the activation of the C-H bond of alkyne by nano-catalyst. The generated catalyst-acetylide intermediate subsequently reacts with the iminium ion generated in situ from catalyst activated 1 and 2 to give the corresponding propargylamine 4 (Scheme 2). In particular, the reaction proceeds via the initial formation of an iminium intermediate from the starting amine and aldehyde. However, the iminium formation is equilibrated, and this equilibrium could be influenced by the catalyst. The presence of Fe3O4 NPs in the catalyst composition could indeed favor iminium formation. Iron cations acts as Lewis acid and play a significant role in increasing the electrophilic character of the starting aldehyde and stabilizing the immonium salt by the coordination of the oxygen or nitrogen lone electron pair [14]. Moreover, there are some reports on iron-catalyzed coupling of aldehyde, alkyne and amine which proposed the generation of terminal iron-acetylide as a tentative mechanism of the reaction [23]. We examined the role of Fe3O4 NPs through performing the reaction by synthesized GO-CuO NPs nanocatalyst (without Fe3O4 NPs). In the optimum conditions only 53% yield is obtained showing the important role of magnetite NPs in the efficiency of catalyst. The copper ions present in the Fe3O4 NPs/ GO-CuO NPs act as the C-H activation catalyst. Upon coordination, the alkyne is probably deprotonated, and copper-acetylide thus formed which could then add concomitantly to the iminium compound produced within the Fe3O4 NPs/GO [38].
|
Download:
|
| Scheme 2.Tentative mechanism for the A3-coupling reaction catalyzed over Fe3O4 NPs/GO-CuO NPs, where small spheres (●) represent CuO NPswhile larger ones (●) indicate Fe3O4 NPs. | |
Ease of separation and possible reusability is an important factor in the heterogeneous catalyst effectiveness. In order to increase the Fe3O4 NPs/GO-CuO NPs efficiency and decrease waste, the recycle ability of the catalyst is investigated. The catalyst is isolated simply using an external magnet and washed with ethanol and after air-drying it reused directly for the next trial. The yield of the reaction decreases by 3% in the first run, while this amount is less than 6% detected in the next three cycles, indicating the high efficiency of the recovered Fe3O4 NPs/GO-CuO NPs catalyst (Fig. 2).

|
Download:
|
| Fig. 2.Yields obtained with recycledFe3O4 NPs/GO-CuO NPs catalyst. | |
An efficient Fe3O4 NPs/GO-CuO NPs-catalyzed three-component onepot coupling of aldehydes, amines, and alkynes has been achieved. The process is simple and generates a diverse range of propargylamines in good yields. The reaction has high atom efficiency, since water is the only byproduct. All these facts together with easy work-up and clean reaction profile, a wide scope of the substrates, and cost effectiveness of the catalyst permit us to anticipate a good future for this protocol not only in academia but perhaps in industry.
| [1] | C.M. Wei, Z.G. Li, C.-J. Li, The development of A3-coupling (aldehyde-alkyne-amine) and AA3-coupling (asymmetric aldehyde-alkyne-amine), Synlett (2004) 1472-1483. |
| [2] | L. Zani, C. Bolm, Direct addition of alkynes to imines and related C=N electrophiles:a convenient access to propargylamines, Chem. Commun. (2006) 4263-4275. |
| [3] | C.M. Wei, Z.G. Li, C.-J. Li, The first silver-catalyzed three-component coupling of aldehyde, alkyne, and amine, Org. Lett. 5(2003) 4473-4475. |
| [4] | V.A. Peshkov, O.P. Pereshivko, E.V. Van der Eycken, A walk around the A3-coupling, Chem. Soc. Rev. 41(2012) 3790-3807. |
| [5] | W. Shi, C. Liu, A.W. Lei, Transition-metal catalyzed oxidative cross-coupling reactions to form C-C bonds involving organometallic reagents as nucleophiles, Chem. Soc. Rev. 40(2011) 2761-2776. |
| [6] | A.A. Boulton, B.A. Davis, D.A. Durden, et al., Aliphatic propargylamines:new antiapoptotic drugs, Drug Dev. Res. 42(1997) 150-156. |
| [7] | E.-S. Lee, H.-S. Yeom, J.-H. Hwang, et al., A practical gold-catalyzed route to 4-substituted oxazolidin-2-ones from N-boc propargylamines, Eur. J. Org. Chem. 2007(2007) 3503-3507. |
| [8] | A. Kochman, J. Skolimowski, L. Gêbicka, D. Metodiewa, Antioxidant properties of newly synthesized N-propargylamine derivatives of nitroxyl:a comparison with deprenyl, Pol. J. Pharmacol. 55(2003) 389-400. |
| [9] | F.P. Xiao, Y.L. Chen, Y. Liu, J.B. Wang, Sequential catalytic process:synthesis of quinoline derivatives by AuCl3CuBr-catalyzed three-component reaction of aldehydes, amines, and alkynes, Tetrahedron 64(2008) 2755-2761. |
| [10] | D. Shibata, E. Okada, J. Molette, M. Médebielle, Facile synthesis of fluorinecontaining 1, 10-phenanthrolines by the pyridine-ring formation reaction of Npropargyl-5, 7-bis(trifluoroacetyl)-8-quinolylamine with amines:isolation of the intermediates 1, 4-dihydro-1, 10-phenanthrolin-4-ols, Tetrahedron Lett. 49(2008) 7161-7164. |
| [11] | D.F. Harvey, D.M. Sigano, Synthesis of cyclopropylpyrrolidines via reaction of Nallyl-N-propargylamides with a molybdenum carbene complex:effect of substituents and reaction conditions, J. Org. Chem. 61(1996) 2268-2272. |
| [12] | B. Yan, Y.H. Liu, Gold-catalyzed multicomponent synthesis of aminoindolizines from aldehydes, amines, and alkynes under solvent-free conditions or in water, Org. Lett. 9(2007) 4323-4326. |
| [13] | M.E. Jung, A. Huang, Use of optically active cyclic N, N-dialkyl aminals in asymmetric induction, Org. Lett. 2(2000) 2659-2661. |
| [14] | R. Bloch, Additions of organometallic reagents to C=N bonds:reactivity and selectivity, Chem. Rev. 98(1998) 1407-1438. |
| [15] | S. Samai, G.C. Nandi, M.S. Singh, An efficient and facile one-pot synthesis of propargylamines by three-component coupling of aldehydes, amines, and alkynes via C-H activation catalyzed by NiCl2, Tetrahedron Lett. 51(2010) 5555-5558. |
| [16] | C.M. Wei, C.-J. Li, Enantioselective direct-addition of terminal alkynes to imines catalyzed by copper (I) pybox complex in water and in toluene, J. Am. Chem. Soc. 124(2002) 5638-5639. |
| [17] | V.K.-Y. Lo, Y.G. Liu, M.-K. Wong, C.-M. Che, Gold (III) salen complex-catalyzed synthesis of propargylamines via a three-component coupling reaction, Org. Lett. 8(2006) 1529-1532. |
| [18] | J.X. Ji, T.T.-L. Au-Yeung, J. Wu, C.W. Yip, A.S.C. Chan, Efficient synthesis of β, γ-alkynyl α-amino acid derivatives by Ag (I)-catalyzed alkynylation of α-imino esters, Adv. Synth. Catal. 346(2004) 42-44. |
| [19] | N. Gommermann, P. Knochel, Preparation of functionalized primary chiral amines and amides via an enantioselective three-component synthesis of propargylamines, Tetrahedron 61(2005) 11418-11426. |
| [20] | N.G. Dipl.-Chem, C. Koradin, K. Polborn, P. Knochel, Enantioselective, copper (I)-catalyzed three-component reaction for the preparation of propargylamines, Angew. Chem. Int. Ed. 42(2003) 5763-5766. |
| [21] | G.W. Kabalka, L.-L. Zhou, L. Wang, R.M. Pagni, A microwave-enhanced, solventless Mannich condensation of terminal alkynes and secondary amines with paraformaldehyde on cuprous iodide doped alumina, Tetrahedron 62(2006) 857-867. |
| [22] | C. Fischer, E.M. Carreira, Zn-alkynylide additions to acyl iminiums, Org. Lett. 6(2004) 1497-1499. |
| [23] | W.-W. Chen, R.V. Nguyen, C.-J. Li, Iron-catalyzed three-component coupling of aldehyde, alkyne, and amine under neat conditions in air, Tetrahedron Lett. 50(2009) 2895-2898. |
| [24] | J.S. Yadav, B.V. Subba Reddy, A.V. Hara Gopal, K.S. Patil, InBr3-catalyzed threecomponent reaction:a facile synthesis of propargyl amines, Tetrahedron Lett. 50(2009) 3493-3496. |
| [25] | Y.C. Zhang, P.H. Li, M. Wang, L. Wang, Indium-catalyzed highly efficient threecomponent coupling of aldehyde, alkyne, and amine via C-H bond activation, J. Org. Chem. 74(2009) 4364-4367. |
| [26] | C. Fischer, E.M. Carreira, Direct addition of TMS-acetylene to aldimines catalyzed by a simple, commercially available Ir (I) complex, Org. Lett. 3(2001) 4319-4321. |
| [27] | P.H. Li, L. Wang, Mercurous chloride catalyzed Mannich condensation of terminal alkynes with secondary amines and aldehydes, Chin. J. Chem. 23(2005) 1076-1080. |
| [28] | L.C. Akullian, M.L. Snapper, A.H. Hoveyda, Three-component enantioselective synthesis of propargylamines through Zr-catalyzed additions of alkyl zinc reagents to alkynylimines, Angew. Chem. Int. Ed. 42(2003) 4244-4247. |
| [29] | Y. Kuninobu, P. Yu, K. Takai, Rhenium-catalyzed[2+2] cycloadditions of norbornenes with internal and terminal acetylenes, Chem. Lett. 36(2007) 1162-1163. |
| [30] | C.-J. Li, The development of catalytic nucleophilic additions of terminal alkynes in water, Acc. Chem. Res. 43(2010) 581-590. |
| [31] | D. Astruc, Nanoparticles and Catalysis, Wiley-VCH, Weinheim, 2008. |
| [32] | V. Polshettiwar, R.S. Varma, Green chemistry by nano-catalysis, Green Chem. 12(2010) 743-754. |
| [33] | R.J. White, R. Luque, V.L. Budarin, J.H. Clark, D.J. Macquarrie, Supported metal nanoparticles on porous materials. Methods and applications, Chem. Soc. Rev. 38(2009) 481-494. |
| [34] | P.R. Likhar, S. Roy, M. Roy, et al., Silica-immobilized CuI:an efficient reusable catalyst for three-component coupling reaction of aldehyde, amine and alkyne, Synlett 2007(2007) 2301-2303. |
| [35] | B. Sreedhar, P. Surendra Reddy, C.S. Vamsi Krishna, P. Vijaya Babu, An efficient synthesis of propargylamines using a silica gel anchored copper chloride catalyst in an aqueous medium, Tetrahedron Lett. 48(2007) 7882-7886. |
| [36] | M.L. Kantam, J. Yadav, S. Laha, et al., Synthesis of propargylamines by threecomponent coupling of aldehydes, amines and alkynes catalyzed by magnetically separable copper ferrite nanoparticles, Synlett 2009(2009) 1791-1794. |
| [37] | M. Kidwai, V. Bansal, N.K. Mishra, A. Kumar, S. Mozumdar, Copper-nanoparticlecatalyzed A3coupling via CH activation, Synlett 2007(2007) 1581-1584. |
| [38] | M. Lakshmi Kantam, S. Laha, J. Yadav, S. Bhargava, An efficient synthesis of propargylamines via three-component coupling of aldehydes, amines and alkynes catalyzed by nanocrystalline copper (II) oxide, Tetrahedron Lett. 49(2008) 3083-3086. |
| [39] | M.J. Aliaga, D.J. Ramó n, M. Yus, Impregnated copper on magnetite:an efficient and green catalyst for the multicomponent preparation of propargylamines under solvent free conditions, Org. Biomol. Chem. 8(2010) 43-46. |
| [40] | P.D. Stevens, G.F. Li, J.D. Fan, M. Yen, Y. Gao, Recycling of homogeneous Pd catalysts using superparamagnetic nanoparticles as novel soluble supports for Suzuki, Heck, and Sonogashira cross-coupling reactions, Chem. Commun. (2005) 4435-4437. |
| [41] | J.H. Deng, X.H. Wen, Q.N. Wang, Solvothermal in situ synthesis of Fe3O4-multiwalled carbon nanotubes with enhanced heterogeneous Fenton-like activity, Mater. Res. Bull. 47(2012) 3369-3376. |
| [42] | A. Prakash, S. Chandra, D. Bahadur, Structural, magnetic, and textural properties of iron oxide-reduced graphene oxide hybrids and their use for the electrochemical detection of chromium, Carbon 50(2012) 4209-4219. |
| [43] | T. Zeng, X.L. Zhang, Y.R. Ma, H.Y. Niu, Y.Q. Cai, A novel Fe3O4-graphene-Au multifunctional nanocomposite:green synthesis and catalytic application, J. Mater. Chem. 22(2012) 18658-18663. |
| [44] | M.Z. Kassaee, E. Motamedi, B. Movassagh, S. Poursadeghi, Iron-catalyzed formation of C-Se and C-Te bonds through cross coupling of aryl halides with Se (0) and Te (0)/Nano-Fe3O4@GO, Synthesis 45(2013) 2337-2342. |
| [45] | M.Z. Kassaee, E. Motamedi, M. Majdi, Magnetic Fe3O4-graphene oxide/polystyrene:fabrication and characterization of a promising nanocomposite, Chem. Eng. J. 172(2011) 540-549. |
| [46] | W.S. Hummers Jr., R.E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc. 80(1958) 1339. |
| [47] | S. Stankovich, R.D. Piner, S.T. Nguyen, R.S. Ruoff, Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets, Carbon 44(2006) 3342-3347. |
 2015, Vol.26
2015, Vol.26