b Department of Pharmaceutical and Biological Engineering, College of Chemical Engineering, Sichuan University, Chengdu 610065, China
Spiropyrrolidine oxindoles are important structural skeletons in organic synthesis because of their versatile utility as building blocks common to many natural isolates [1, 2, 3, 4, 5] and other unnatural useful compounds. Many diverse spiropyrrolidine oxindole ring system especially spiro[pyrrolidin-2,30 -oxindoles] possessed interesting structural properties and highly pronounced pharmacological activity,including anticancer activity [6, 7, 8],cholinesterase enzyme inhibitor [9],advanced glycation end product formation inhibitory activity [10],antimicrobial activity [11, 12] and antidiabetic activity [13]. The significance in bioactivity profiles may be due to their unique structural features,whose structural frameworks assemble two important privileged motifs into one molecule in a spiro manner,through an intriguing fusion at the C3-position of the oxindole core with the construction of pyrrolidine ring. As an attractive synthetic target,intense efforts have been made on the development of various elegant and efficientmethods to access the structurally diverse family of spiro[pyrrolidin-2,30 -oxindoles] dur- ing the past few years,examples of the representative methods include 1,3-dipolar cycloaddition [14, 15, 16, 17, 18],nucleophilic addition [19, 20, 21],intramolecular hydroamination [22],homoenolate addi- tion [23, 24],Buchwald-Hartwig/Michael reaction [25],aromatiza- tion [26],reductive cyclization [27],and other strategies. Although a great improvement has beenmade in synthesis strategies,given the potential bioactive and medicinal significance of spiro[pyrrolidin- 2,30 -oxindoles],it is still important and desirable to develop more effectiveandcreativemethods,including thedesignof substrate and methodology itself,which is still a very challenging task.
Tandem reactions,or so-called domino reactions,are powerful tools for rapidly achieving molecular complexity,which not only constructs more than two new bonds by one chemical operation but also omits many perplexing reaction steps and shortens the operations. As regards classical methodologies,the Michael addition is one of the most fundamental and powerful carbon- carbon bond-constructing methods,playing an increasing signifi- cant role as part of tandemreactions in organic synthesis especially in the construction of novel ring [28, 29, 30]. As newly emerged nucleophiles,some reports have shown that 3-monosubstituted 3- aminooxindoles can be used to prepare 3,3-disubstituted oxi- ndoles with the help of Michael addition [31, 32]. However,to the best of our knowledge,no examples with 3-aminooxindoles hydrochloride as nucleophiles for cascade Michael/cyclization reactions were reported. As part of our continuing interest in the development of novel synthetic methods in polycyclic hetero- cycles,we try hard to complement a new strategy,as a candidate reference method,for the preparation of diverse 3',4'-dihydros- piro[pyrrol-3,20-oxindoles] through a tandem Michael addition- cyclization process (Scheme 1). Herein,we report our preliminary progress in this area for the preparation of biologically important 3',4'-dihydrospiro[pyrrol-3,20-oxindoles] with acceptable results.
|
Download:
|
| Scheme. 1. Domino strategy for the synthesis of 3’,4’-dihydrospiro[indoline-3,20-pyrrol]-2-ones 4. | |
All the chemical reagents in this work were acquired from different commercial sources and used without further purifica- tion. All reactions were monitored by thin layer chromatography (TLC) with 60 F254 silica gel plates. All products were purificated by using column chromatography (200-300 mesh silica gel, Qingdao Marine Chemical Ltd.,Qingdao,China). The 1 H NMR spectra were recorded on a Bruker AM400 NMR spectrometer and the chemical shifts in ppm were reported relative to tetramethyl- silane (TMS) or residual solvent peaks. High-resolution mass spectrometry (HRMS) data of the synthesized compounds were recorded using a Waters Q-Tof premier mass spectrometer.
Typical experimental procedure for the synthesis of 30 ,40 - dihydrospiro[indoline-3,2’ -pyrrol]-2-one derivatives 4: A reac- tion tube equipped with a magnetic stirrer bar was charged with 3-aminoindolin-2-ones hydrochloride 1 (0.3 mmol),enones/ enals 2 (0.3 mmol) and ethylene glycol (3 mL),and then DBU 3a(114.18 mg,0.75 mmol) was added to the reaction mixture. The reaction tube was placed in an oil bath at 100 8Candstirred for 1 h. After completion of the reaction (TLC),the crude product was purified by column chromatography on silica gel using dichloromethane/ethyl acetate (9/1) as the eluent to give product 4.
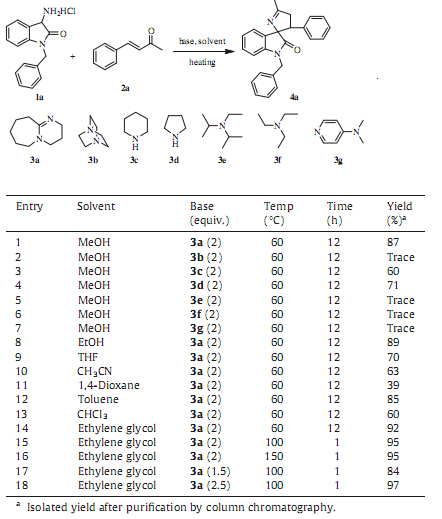
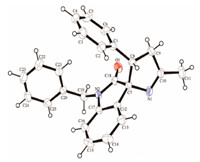
3. Results and discussionThe studies were initiated by evaluating the domino reaction between 3-amino-1-benzylindolin-2-one hydrochloride 1a as a nucleophile and benzalacetone 2a as an acceptor in methanol at 60 8C in the presence of 2 equiv. DBU,which played a dual role in reaction: one was the alkalization of 3-amino-1-benzylindolin-2- one hydrochloride releasing free 3-amino-1-benzylindolin-2-one, the other was the catalysis of this cascade Michael/cyclization reaction. The reaction took around 12 h and proceeded smoothly to afford the desired compound 4a in 87% isolated yield (Table 1, entry 1). The structure of compound 4a was confirmed by mass spectrometry analysis,NMR analysis and single crystal X-ray crystallographic study (Fig. 1).
|
Download:
|
| Fig. 1. X-ray crystal structure of compound 4a.Crystallographic data have been deposited with CCDC (No. CCDC-1001212). | |
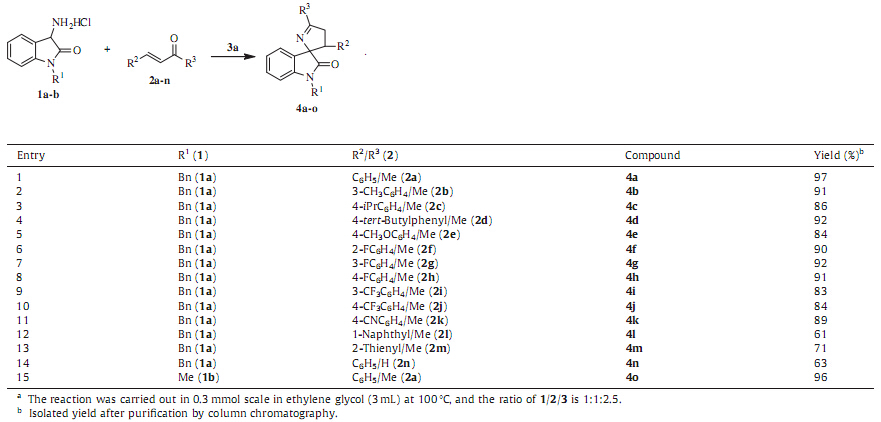
This model reaction was then catalyzed by other organic bases such as triethylenediamine,piperidine,pyrrolidine,ethyldiisopro- pylamine,triethylamine and 4-dimethylaminopyridine,to evalu- ate the influence of different organic bases on the yield and screen out the ideal organic base with best performance. To our disappointment,the reactions with other organic bases showed inferior results in terms of the yield compared with DBU as the catalyst (Table 1,entries 2-7). As a result,DBUwas selected as ideal organic base in further screening of other reaction parameters including solvent,reaction temperature and the amount of base.Under the analogous conditions,various regular organic solvents such as methanol,ethanol,tetrahydrofuran,acetonitrile,1,4- dioxane,toluene,chloroform,and ethylene glycol were explored and the results were summarized in Table 1 (entries 1,8-14). Experimental results demonstrated that the reaction in three alcoholic solvents proceeded smoothly in better yield than that of other solvents,and the efficacy of the alcoholic solvents assayed changed in the order:methanol < ethanol < ethylene glycol. Then, we turned our attention to reaction temperature,expecting that the yield may increase with a rise in temperature (Table 1,entries 14-16). The results revealed that 100 8C was sufficient to complete this reaction shortening the reaction time to 1 h,and higher temperature was no help for the improvement of the yield. We further explored the effects of the amount of DBU,which had significant influence on the yield (Table 1,entry 15 and entries 17- 18). Through extensive screening,we have come to conclude that the reaction proceeded most efficiently in the presence of 2.5 equiv. of DBU in ethylene glycol at 100 8C for 1 h,and the desired product was isolated in 97% yield (Table 1,entry 18).
| Table 1 Optimization of reaction conditions. |
Having identified the optimal reaction conditions in hand,we directed our efforts to explore the substrate scope of the reaction with respect to the use of different protected 3-aminooxindoles and enones/enals (Table 2). It was observed that regardless of whether an electron-donating group (Table 2,entries 2-5) or an electron-withdrawing group (Table 2,entries 6-11) was contained on the phenyl ring of the enones,the reactions proceeded well, affording the products 4b-k with 83%-92% isolated yields. Afterward,1-naphthyl-based enone 2l also gave rise to the product 4l in acceptable yield (Table 2,entry 12). And heterocyclic analog 2m was also successfully employed to react with 3-amino-1- benzylindolin-2-one hydrochloride 1a,furnishing 4m in 71% isolated yield (Table 2,entry 13). Moreover,the possibility of using other enals as the Michael acceptor was also studied to further extend the application of our procedure (Table 2,entry 14). In the end,replacing the benzyl moiety on the N-1 of 3-amino-1- benzylindolin-2-one hydrochloride 1a with amethyl group had no significant effect on the reaction,and the corresponding product 4o could be achieved in 96% isolated yield (Table 2,entry 15).
| Table 2 Synthesis of 3’,4’-dihydrospiro[indoline-3,2’-pyrrol]-2-one derivatives.. |
In conclusion,we have developed a new strategy for the construction of potentially biologically active 3’ ,4’ -dihydrospir- o[pyrrol-3,2’ -oxindoles] through a cascade Michael/cyclization reaction of 3-aminoindolin-2-ones with enones/enals. There was no obvious impact on efficiency of the reaction,regardless of the electronic nature,bulkiness,or position of the substituent in the phenyl ring of enones. The present methodology provides a direct and efficient method for the preparation of more diverse 3’ ,4’ -dihydrospiro[pyrrol-3,2’ -oxindoles] analog libraries in mod- erate to high yields (up to 97%).
Acknowledgments
Financial support for this work by the National Natural Science Foundation of China (No. 81202403) and West China Hospital- Chengdu Science and Technology Department Translational Medicine Innovation Foundation (No. ZH13039) are gratefully acknowledged. We also thank Sichuan University Analytical & Testing Center for the NMR analysis.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.014.
| [1] | R. Rojas-Duran, G. Gonzalez-Aspajo, C. Ruiz-Martel, et al., Anti-inflammatory activity of mitraphylline isolated from Uncaria tomentosa bark, J. Ethnopharmacol. 143 (2012) 801-804. |
| [2] | I. Gulcin, S. Beydemir, F. Topal, et al., Apoptotic, antioxidant and antiradical effects of majdine and isomajdine from Vinca herbacea Waldst. and kit, J. Enzyme Inhib. Med. Chem. 27 (2012) 587-594. |
| [3] | J.H. Lu, J.Q. Tan, S.S.K. Durairajan, et al., Isorhynchophylline, a natural alkaloid, promotes the degradation of α-synuclein in neuronal cells via inducing autophagy, Autophagy 8 (2012) 98-108. |
| [4] | G.G. Cheng, Y.L. Zhao, Y. Zhang, et al., Indole alkaloids from cultivated Vinca major, Tetrahedron 70 (2014) 8723-8729. |
| [5] | H.J. Cong, Q. Zhao, S.W. Zhang, et al., Terpenoid indole alkaloids from Mappianthus iodoides Hand. -Mazz., Phytochemistry 100 (2014) 76-85. |
| [6] | Y. Arun, K. Saranraj, C. Balachandran, et al., Novel spirooxindole-pyrrolidine compounds: synthesis, anticancer and molecular docking studies, Eur. J. Med. Chem. 74 (2014) 50-64. |
| [7] | J.M. Yang, Y. Hu, Q. Li, et al., Efficient and regioselective synthesis of novel functionalized dispiropyrrolidines and their cytotoxic activities, ACS Comb. Sci. 16 (2014) 139-145. |
| [8] | B. Yu, X.J. Shi, P.P. Qi, et al., Design, synthesis and biological evaluation of novel steroidal spiro-oxindoles as potent antiproliferative agents, J. Steroid Biochem. Mol. Biol. 141 (2014) 121-134. |
| [9] | Y. Kia, H. Osman, R.S. Kumar, et al., Synthesis and discovery of highly functionalized mono-and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors, Bioorg. Med. Chem. Lett. 24 (2014) 1815-1819. |
| [10] | A. Kaur, B. Singh, B. Vyas, et al., Synthesis and biological activity of 4-aryl-3-benzoyl-5-phenylspiro[pyrrolidine-2.3'-indolin]-2'-one derivatives as novel potent inhibitors of advanced glycation end product, Eur. J. Med. Chem. 79 (2014) 282-289. |
| [11] | A.V. Velikorodov, V.A. Ionova, O.V. Degtyarev, et al., Synthesis and antimicrobial and antifungal activity of carbamate-functionized spiro compounds, Pharm. Chem. J. 46 (2013) 715-719. |
| [12] | Y. Arun, G. Bhaskar, C. Balachandran, et al., Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line, Bioorg. Med. Chem. Lett. 23 (2013) 1839-1845. |
| [13] | R. Murugan, S. Anbazhagan, S.S. Narayanan, Synthesis and in vivo antidiabetic activity of novel dispiropyrrolidines through [3 + 2] cycloaddition reactions with thiazolidinedione and rhodanine derivatives, Eur. J. Med. Chem. 44 (2009) 3272-3279. |
| [14] | R. Rajesh, R. Raghunathan, Synthesis of β-lactam-tethered polycyclic fused heterocycles through a rearrangement by a one-pot tandem [3 + 2] cycloaddition reaction, Eur. J. Org. Chem. 13 (2013) 2597-2607. |
| [15] | K. Suman, L. Srinu, S. Thennarasu, Lewis acid catalyzed unprecedented [3 + 2] cycloaddition yields 3,3'-pyrrolidinyldispirooxindoles containing four contiguous chiral stereocenters with two contiguous quaternary spirostereocenters, Org. Lett. 16 (2014) 3732-3735. |
| [16] | A. Hemamalini, S. Nagarajan, T.M. Das, A novel class of sugar-based ether-linkeddispirooxindolo-pyrrolidines/pyrrolizidines through [3 + 2]-cycloaddition of azomethine ylides, Carbohydr. Res. 352 (2012) 12-17. |
| [17] | S. Purushothaman, R. Prasanna, S. Lavanya, et al., Regio-and stereoselective synthesis of spiro-pyrrolidine/pyrrolizidine/thiazolidine-grafted macrocycles through intramolecular 1,3-dipolar cycloaddition reaction, Tetrahedron Lett. 54 (2013) 5744-5747. |
| [18] | H.B. Yang, Y. Wei, M. Shi, Construction of spiro[indoline]oxindoles through onepot thermal-induced [3 + 2] cycloaddition/silica gel-promoted fragmentation sequence between isatin ketonitrones and electron-deficient alkynes, Tetrahedron 69 (2013) 4088-4097. |
| [19] | Y.M. Cao, F.F. Shen, F.T. Zhang, et al., Catalytic asymmetric Michael addition/ cyclization of isothiocyanato oxindoles: highly efficient and versatile approach for the synthesis of 3,2'-pyrrolidinyl mono-and bi-spirooxindole frameworks, Chem. Eur. J. 19 (2013) 1184-1188. |
| [20] | F. Tan, L.Q. Lu, Q.Q. Yang, et al., Enantioselective cascade Michael addition/ cyclization reactions of 3-nitro-2H-chromenes with 3-isothiocyanato oxindoles: efficient synthesis of functionalized polycyclic spirooxindoles, Chem. Eur. J. 20 (2014) 3415-3420. |
| [21] | Y. Sun, J. Sun, C.G. Yan, Synthesis of 1'-aryl-2'-(2-oxoindolin-3-yl)spiro[indoline-3,5'-pyrroline]-2,3'-dione via one-pot reaction of arylamines, acetone, and isatins, Tetrahedron Lett. 53 (2012) 3647-3649. |
| [22] | D. Chen, M.H. Xu, Zn-mediated asymmetric allylation of N-tert-butanesulfinyl ketimines: an efficient and practical access to chiral quaternary 3-aminooxindoles, Chem. Commun. 49 (2013) 1327-1329. |
| [23] | B. Zhang, P. Feng, L.H. Sun, et al., N-Heterocyclic carbene-catalyzed homoenolate additions with N-aryl ketimines as electrophiles: efficient synthesis of spirocyclic γ-lactam oxindoles, Chem. Eur. J. 18 (2012) 9198-9203. |
| [24] | H. Lv, B. Tiwari, J.M. Mo, et al., Highly enantioselective addition of enals to isatinderived ketimines catalyzed by N-heterocyclic carbenes: synthesis of spirocyclic γ-lactams, Org. Lett. 14 (2012) 5412-5415. |
| [25] | N. Sharma, Z.H. Li, U.K. Sharma, et al., Facile access to functionalized spiro[indoline-3,2'-pyrrole]-2,5'-diones via post-Ugi domino Buchwald-Hartwig/Michael reaction, Org. Lett. 16 (2014) 3884-3887. |
| [26] | A. Srivastava, S.M. Mobin, S. Samanta, (+/-)-CSA catalyzed one-pot synthesis of 6,7-dihydrospiro[indole-3,1'-isoindoline]-2,3',4(1H,5H)-trione derivatives: easy access of spirooxindoles and ibophyllidine-like alkaloids, Tetrahedron Lett. 55 (2014) 1863-1867. |
| [27] | M.S. Poslusney, B.J. Melancon, P.R. Gentry, et al., Spirocyclic replacements for the isatin in the highly selective, muscarinic M1 PAM ML137: the continued optimization of an MLPCN probe molecule, Bioorg. Med. Chem. Lett. 23 (2013) 1860-1864. |
| [28] | H.A. Soliman, T.A. Salama, Silicon-mediated highly efficient synthesis of 1,8-dioxo-octahydroxanthenes and their transformation to novel functionalized pyrano-tetrazolo [1,5-a] azepine derivatives, Chin. Chem. Lett. 24 (2013) 404-406. |
| [29] | V.M. Joshi, R.L. Magar, P.B. Throat, et al., Novel one-pot synthesis of 4H-chromene derivatives using amino functionalized silica gel catalyst, Chin. Chem. Lett. 25 (2014) 455-458. |
| [30] | D.C. Wang, Y.M. Xie, C. Fan, et al., Efficient and mild cyclization procedures for the synthesis of novel 2-amino-4H-pyran derivatives with potential antitumor activity, Chin. Chem. Lett. 25 (2014) 1011-1013. |
| [31] | B.D. Cui, W.Y. Han, Z.J. Wu, et al., Enantioselective synthesis of quaternary 3-aminooxindoles via organocatalytic asymmetric Michael addition of 3-monosubstituted 3-aminooxindoles to nitroolefins, J. Org. Chem. 78 (2013) 8833-8839. |
| [32] | B.D. Cui, J. Zuo, J.Q. Zhao, et al., Tandem Michael addition-ring transformation reactions of 3-hydroxyoxindoles/3-aminooxindoles with olefinic azlactones: direct access to structurally diverse spirocyclic oxindoles, J. Org. Chem. 79 (2014) 5305-5314. |