b School of Chemistry & Chemical Engineering, Jiangsu University, Zhenjiang 212013, China;
c School of Chemistry, Jilin Normal University, Siping 136000, China
Pickering high internal phase emulsions (HIPEs) are commonly defined as highly viscous emulsions stabilized by solid where the volume fraction of the dispersed phase exceeds 74.05% [1]. If the continuous phase was polymerizable,HIPEs can be used as templates to prepare porous polymeric materials,known as polyHIPEs [2]. This kind of material possesses a three-level porosity: Larger pores due to the solid stabilized droplets of the non-polymerizable internal phase,smaller interconnecting pores due to the addition of a small amount of surfactant and the porosity of the polymer film itself due to the functions of the pore forming agent [3]. Because of the unique morphology of the polyHIPEs material,they are suitable for a wide range of applications,such as conductive composite foams [4],catalyst supports [5] and adsorbents [6]. Moreover,the HIPEs are also used in membrane separation. Compared with other unit operations,membrane processes can be performed isothermally at low energy consump- tion and easy to be upscaled or downscaled as well as integrated into other separations or reactions [7, 8, 9]. For instance,Ruckenstein and co-workers first studied the application of polyHIPEs thin films for permselective separation of ethanol-water liquidmixtures and some organic solvents [10, 11]. Additionally,there were reports on the preparation of polyHIPEs membranes via a casting method for protein separation [12, 13].
However,these membranes prepared by HIPEs are not sufficiently selective for certain substances. To improve the selectivity,molecular imprinting technology (MIT) is introduced. During molecular imprinting,the template molecule and func- tional monomers first arrange in a certain orientation owing to their interactions controlled by both intermolecular forces and shape complementarity. The preorganized complex is then copolymerized with a crosslinker. After polymerization,the templatemolecule is extracted,leaving a specific site for rebinding the template molecule [14]. Over the last few decades,MIT has been extensively developed and used for detecting various molecular species [15, 16, 17, 18, 19]. This technique offers an opportunity of producing biomimetic materials,which are able to recognize certain molecules based onshape,size and chemical functionality in closely related compound mixtures [20, 21]. Combining the Pickering HIPEs template with the MIT would offer a new method with great potential in the field of separation and analysis of certain molecules. Pan et al. [22] pioneered molecularly imprinted polymer foams (MIPFs) using water-in-oil (W/O) Pickering HIPEs to enhance the recognition of l-cyhalothrin,which showed some promise. However,polyHIPEs membranes in combination with MIT for separating molecules from their structurally related analogs have not been reported yet.
Methyl 4-hydroxybenzoate (M4HB) as a useful preservative is widely used in organic synthesis,cosmetics,medicine and food industries. However,it was suggested that the allergic reactions could occur after parabens ingestion [23]. Besides,several papers reported that M4HB on skin may react with ultraviolet radiation B (UVB),leading to increasing skin aging and DNA damages [24, 25]. Moreover,chronic exposure of M4HB might cause gastrointestinal discomfort,inflammation in skin andmucosa,and female hormone disorders. Therefore,it is important and necessary to make sure that the content ofM4HB in cosmetics and food iswithin the upper limit permitted. The challenge is that the concentration ofM4HB in detected products is extremely low,and it is very difficult to make a selective separation using traditional technologies,such as distillation,extraction,dialysis and so on. Kannathasan et al. [26] isolated a crystalline compound methyl-p-hydroxybenzoate from the methanol extract of Vitex trifolia leaves and purified by silica gel glass column chromatography. However,the process of separation was complex and was not applicable for treatment of most real samples. Therefore,it is important to search for a simple and highly effective method to separate and enrich the target selectively.
In this work,we synthesized the molecularly imprinted open porous membranes (MIOPMs) with a well-defined open-cell structure using water-in-oil (W/O) Pickering HIPEs. MIOPMs were then used to selectively adsorb the template M4HB. The concentration effect of the plastifier 2-ethylhexyl acrylate (2-EHA) on themechanical properties,morphology and imprinting effect were also described. In addition,the mechanism of the membrane adsorption for M4HB was discussed. And the applica- tion of these novel membrane materials for the permeation of M4HB was reported.
2. ExperimentalHydrophobic silica nanoparticles (HNP-SiO2) with diameters of 7-40 nm,methyl 4-hydroxybenzoate (M4HB),methyl 2-hydro- xybenzoate (M2HB),methyl methacrylate (MMA),2-ethyhexyl acrylate (2-EHA),ethylene glycol dimethacrylate (EGDMA) and N,N,N0 ,N0 -tetramethylethylenediamine (TEMED)were bought from Aladdin Chemistry (Shanghai,China) Co.,Ltd. Sorbitantrioleate (Span 85),styrene (St),ammonium persulfate (APS) and calcium chloride anhydrous (CaCl2) were purchased from Sinopharm Chemical Reagent (Shanghai,China) Co.,Ltd. All reagents were used as received. Ultra pure water used throughout the experi- ments was obtained from an in-house laboratory purification system. The molecular structure of the membranes was charac- terized using Fourier transform infrared spectrometer (FTIR, Nicolet Nexus 470,USA) in the wave number range of 4000- 400 cm-1 . The morphology of the MIOPMs was studied by scanning electron microscopy (SEM,JSM-7001F,Japan).
Organic phase (25%) and aqueous phase (75%) were prepared separately. Organic phase consisting of monomers St and MMA,crosslinker EGDMA,template M4HB,plastifier 2-EHA (their mole ratio was listed in Table 1),stabilizer HNP-SiO2 (1 wt% of organic phase) and surfactant span 85 (10 vol% of organic phase) [27] were put into a 100 mL three-necked round-bottomed flask with an overhead stirrer at room temperature. Then the organic phase was mechanically stirred at 400 rpm for 15 min. Aqueous phase containing CaCl2 and APS,whose concentration were 0.2 mol L-1 and 2.0 g L-1 respectively,were added dropwise to the organic phase with a dropper after sonication. In the process of aqueous solution dropping,the transparentmixed liquid becamewhite and increasingly viscous. After the addition of the aqueous solution, stirring was continued for 20 min to produce a homogeneous emulsion. TEMED was added to the emulsion a few seconds before the end of the mixing [13].
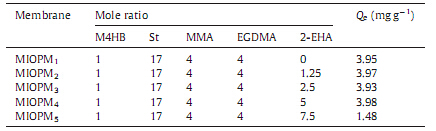
| Table 1 Parameters of the molecularly imprinted W/O pickering HIPEs as well as the adsorption capacity of the MIOPMs. |
The obtained emulsions were cast on the beaker and covered with a preservative film. Then the emulsions were polymerized at room temperature for 24 h to form the polyHIPEs,which were cut into round thin slices using a bladewith dimensioned slits between 300 and 500 mmlater.Afterwards the roundthinsliceswerewashed thoroughlywithmixed solvents ofmethanol/acetic acid (9/1,v/v) to remove non-grafted polymer,residual initiator and the template M4HB by Soxhlet extraction several times. Elution process contin- ued until no template was detected in the eluent by a UV-vis spectrophotometermonitoring the band of 200-400 cm-1 followed by elution of pure methanol. Finally,MIOPMs were obtained by filtrating and drying at room temperature.
As the control,the non imprinted open porous membranes (NIOPMs) were prepared using the same way except for the absence of the template molecule M4HB.
The experiment of adsorption isothermwas studied at 25 8C for 240 min by immersing a 1/4 piece of MIOPMs (about 0.02 g) into 9 mL solutions of methanol/deionized water (3/7,v/v) containing various concentrations of M4HB in beakers. After adsorption,the residual concentrations ofM4HB in the solutions were determined using a UV-vis spectrophotometer at the wavelength of 256 nm. The equilibrium adsorption capacity Qe (mg g-1 ) was calculated according to the following equation:
where C0 and Ce (mg L-1 ) are the initial and equilibrium concentrations of M4HB,respectively. V (mL) and m (g) are the volume of the solution and the weight of the MIOPMs or NIOPMs, respectively.In the adsorption kinetic studies,the initial concentration was set at 200 mg L-1 . The concentration of M4HB in the testing solutions was determined at predetermined time intervals. The amount ofM4HB adsorption (Qt,mgg-1 ) was calculated according to the Eq. (1) by changing the equilibrium to certain time t (min).
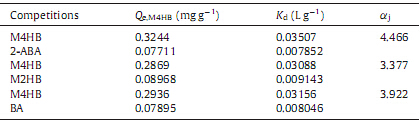
In order to estimate the selectivity of membranes,2-acetox- ybenzoic acid (2-ABA),methyl 2-hydroxybenzoate (M2HB) and benzoic acid (BA) were chosen to evaluate the performance of MIOPMs. The membranes were put into the 50 mL beakers containing 10 mL binary mixed solutions of M4HB and 2-ABA,M4HB and M2HB,M4HB and BA respectively. The tests were carried out at the temperature of 25 8C for 4 h. The distribution coefficients (Kd),selectivity coefficients (aj) of M4HB with regard to 2-ABA,M2HB and BA were calculated according to Eqs. (2) and (3):
where Kd (mL g-1 ) is the distribution coefficient,Qe (mg g-1 ) and Ce (mg L-1 ) represent the bound quantity and the concentration of the adsorbate at equilibrium,respectively: where Kdj represents the distribution coefficient of the competing species.In the permeation experiment,the circular membrane with an effective area of 1.5 cm2 was fixed in an H-shaped two compart- ment cells firmly,whose volume was 150 mL each [28]. Themixed solutions with the volume of 100 mL,which contained 100 mg L-1 of M4HB and M2HB,was placed in the left hand chamber,using as the feeding cell. 100 mL of methanol/water (3/7,v/v) pure solvent was place in the right hand chamber,using as the receiving cell. Both compartment cells were stirred through a magnetic stirrer with a constant rate at 25 8C. After a certain time interval,the amount of M4HB and M2HB in the receiving cell was determined by a UV-vis spectrophotometer. Average permeation amount Qpt (mg cm-2 ) of the substance at any time was calculated by following equation:
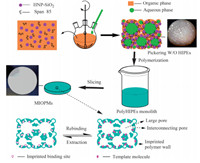
where Ct (mg L-1 ) represents the concentration of the permeated molecule at any time,V (mL) is the volume of the solvent,and A (cm2 ) is the valid surface area of the membrane. To evaluate the permeation selectivity of the obtained membranes,a separation factor (ap) was chosen as a parameter,which was defined as the ratio of the average permeation amount of M4HB and M2HB. 3. Results and discussionThe procedure of preparing the MIOPMs contained two steps: formation of Pickering W/O HIPEs and membrane casting,which are shown in Scheme 1. In the first step,HNP-SiO2 was chosen to stabilize the HIPEs with a small amount of surfactant Span 85. The speed of mechanical stirring should be kept high and the addition of the aqueous phase should be kept slowto avoid demulsification. In the second step,PickeringW/O HIPEs were casted onto a beaker to polymerize at room temperature. The obtained polyHIPEs monolith was then sliced into pieces whose thickness was about 366 mm by measuring the cross-section of MIOPM4 (Fig. 1g). After the removal of the imprinted template,binding sites with stereochemical complementarity to the template molecules were obtained in the as-prepared MIOPMs.
|
Download:
|
| Scheme.1 Formation of MIOPMs derived from PickeringW/O HIPEs and molecular imprinting technology. | |

|
Download:
|
| Fig. 1. SEM Images of MIOPM1-5 (a-e) with different molar ratio of 2-EHA,NIOPM4 (f) and the cross section of MIOPM4 (g). | |
In this study,2-EHA was added to the organic phase of the emulsions to improve the mechanical properties of the membrane material with regard to flexibility. No significant decrease of emulsion stability was observed with different 2-EHA contents, whose molar ratios to M4HB were 0,1.25,2.5,5 and 7.5 for MIOPM1,MIOPM2,MIOPM3,MIOPM4 and MIOPM5,respectively (Table 1). And the more of 2-EHA was added,the more flexible the membranes became. Furthermore,the influence of different 2-EHA contents on the structure of membranes was shown in Fig. 1. As shown,the open-cells with interconnecting and asymmetric pores could be found in MIOPM1-4,the higher the proportion of 2-EHA,the more closed structure,in terms of reducing size of large pores and amount of interconnecting pores,was obtained. And the large poreswere virtually no longer present by incorporation 7.5 equiva- lents of 2-EHA in Table 1 (MIOPM5). In addition,the structure of MIOPM4 (Fig. 1d)was similar to that of NIOPM4 (Fig. 1f),suggesting that the imprinting process did not significantly change the structure of polyHIPEs.
The influence of different 2-EHA content on the adsorption of M4HB was studied using MIOPM1-5 separately to adsorb M4HB molecules in a 10 mL solution containing 200 mg L-1 ofM4HB. The adsorption capacity of the fivemembraneswas list in Table 1. From the data in Table 1,it could be found that the adsorption capacity of the MIOPM1-4 was almost the same except MIOPM5. The reason why the amount of M4HB adsorption by MIOPM5 decreased could be that the structural collapse (Fig. 1g) prevented M4HB from getting inside the membrane. Therefore,incorporation of 2- EHAwith a molar ratio up to 5 in this study was the optimum. The flowing studies used MIOPM4 and NIOPM4 as the testing objects.
The FTIR ofMIOPM4 was shown in Fig. 2. As shown in Fig. 2,the absorption bands around 471 cm-1 and 948 cm-1 were attributed to the symmetric stretching mode of Si-O and the bending vibration modes of free silanol group respectively. The bands observed around 701 cm-1 and 760 cm-1 were assigned to the aromatic stretching vibrations of styrene units. The absorption band at 1726 cm-1 was fromthe carbonyl fromthe esters of 2-EHA and MMA. The result of FTIR indicated that the MIOPMs were prepared successfully.

|
Download:
|
| Fig. 2.FTIR spectra of MIOPM4. | |
In the adsorption isotherm experiment,three nonlinear isotherms including two two-parameter (Langmuir,Freundlich) and one three-parameter (Sips) models were tested. The Langmuir adsorption model further assumes that all the adsorption sites are energetically identical and adsorption occurs on a structurally homogeneous adsorbent,while the Freundlich isotherm model assumes that adsorption occurs on a heterogeneous adsorption surface having sites with different energies of adsorption. The nonlinear form of the Langmuir and Freundlich isotherm models are expressed by the following equations [29, 30]:
where Qe (mg g-1 ) is the equilibrium adsorption capacity and Qm (mg g-1 ) is themaximumamount of theM4HBmolecule adsorbed per unit weight of the adsorbent. Ce is the equilibrium concentra- tion of M4HB (mg L-1 ),and KL is the affinity constant (L mg-1 ): where KF (mg g-1 ) is the adsorption equilibrium constant,and the 1/n is the Freundlich constant.The Sips isotherm model is obtained by introducing a power law expression of the Freundlich isotherm into the Langmuir isotherm and is therefore also called as Langmuir-Freundlich (Sips) isotherm. The nonlinear form of Sips isotherm model can be given as follows:
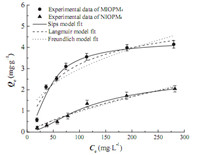
where Ks [(L mg-1 ) ns ] is the Sips isotherm constant representing the energy of adsorption. ns is the empirical constant.The adsorption isothermconstants forMIOPM4 and NIOPM4 are listed in Table 2 and the equilibrium data and modeling for the adsorption ofM4HB ontoMIOPM4 and NIOPM4 are shown in Fig. 3.From Fig. 3,it could be observed that the adsorption capacity increased with the augment of M4HB concentration. The maxi- mum adsorption capacity for MIOPM4 and NIOPM4 were 4.146 mg g-1 and 2.046 mg g-1 respectively when Ce was in the range of 0-300 mg L-1 . Furthermore,from a comparison of the values of R2 in Table 2,it was found that the Sips isotherm model fitted the equilibrium data significantly better than the Langmuir and Freundlich models,indicating that the heterogeneous membrane surface possessing a definite number of adsorption sites.
|
Download:
|
| Fig. 3.Equilibriumdata andmodeling for the adsorption of M4HB ontoMIOPM4 and NIOPM4. | |
| Table 2 Adsorption equilibrium constants for Langmuir,Freundlich and Sips isotherm equations. |
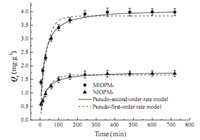
In adsorption kinetic studies,the M4HB adsorption by MIOPM4 and NIOPM4 in various contact time is provided in Fig. 4. The adsorption consisted of both fast and slow reaction processes. The fast process was completed in about 100 min,in which adsorption increased rapidly with contact time,while the slow process could extend to over 6 h,in which the increase of the M4HB adsorption was reduced. Initially,the rate of adsorption was rapid due to the sorption of M4HB onto the exterior surface and may also be attributed to the presence of a large number of binding sites for adsorption. Subsequently,the M4HB molecules entered into the pores,which was a slow process,and the slower adsorption rate was due to the saturation of the binding sites and attainment of equilibrium. Moreover,it could be observed that MIOPM4 exhibited a much higher equilibrium capability and faster transfer than those of NIOPM4,which could be attributed to the imprinting effect of MIOPM4.
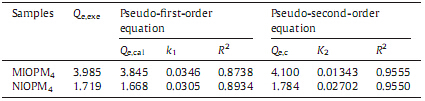
In order to further understand the observed adsorption kinetics ofM4HB on theMIOPM4 and NIOPM4 surfaces,the kinetic datawas analyzed using the pseudo-first-order rate and the pseudo-second- order rate equations,respectively [31, 32]. The pseudo-first-order equation is described as Eq. (8):
The pseudo-second-order equation can be expressed as Eq. (9):
where Qe and Qt are the amount ofM4HB (mg g-1 ) adsorbed on the samples at the equilibriumand time t (min),respectively. k1 and k2 are the equilibrium rate constant for pseudo-first-order (min-1 ) and pseudo-second-order (g mg-1 min-1 ) kinetics,respectively.The modeling for the adsorption of M4HB onto MIOPM4 and NIOPM4 is also shown in Fig. 4,and the adsorption rate constants and linear regression values from two kinetic equations are summarized in Table 3. From Fig. 4,it could be seen that the curve of the pseudo-second order equation fitted well with the experimental data. Furthermore,the correlation coefficient (R2 ) values for the pseudo-second-order kinetic equationwere found to be noticeably higher than those of the pseudo-first-order equation, and the calculated Qe values (Qe,cal) from the pseudo-second-order kinetic equation agreed well with the experimental data (Qe,exp) (Table 3). These results indicated that the adsorption ofM4HB onto MIOPM4 followed the pseudo-second-order kinetic model and the chemical process could be the rate-limiting step in the adsorption process.
|
Download:
|
| Fig. 4.Kinetic data and modeling for the adsorption of M4HB onto MIOPM4 and NIOPM4. | |
| Table 3 Kinetic constants for the pseudo-first-order and pseudo-second-order equations. |
In the static selective adsorption of the membranes,the uptake of M4HB and three competing analogs by MIOPM4 and NIOPM4 as well as distribution coefficients (Kd) and selectivity coefficient (aj) were recorded in Table 4. As listed in Table 4,the MIOPM4 exhibited a higher adsorption value for M4HB than the other competing agents due to the existence of the imprinted cavities that in terms of the shape and size were mutually complementary with M4HB. The value of a2-ABA and aBA was higher than that of aM2HB. This might be explained by the fact that M4HB and M2HB have the same molecular weight and their structures are similar. Therefore,the M2HB was used as the competing agent to further evaluate the permeation selectivity of MIOPM4.
| Table 4 Adsorption selectivity parameters of MIOPM4. |
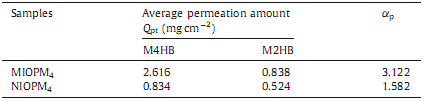
The dependence of permeation amount of M4HB and M2HB through the two membranes on time is shown in Fig. 5. According to the time-dependent permeation curves on the NIOPM4 in Fig. 5(b),the amount ofM4HB andM2HB through the NIOPM4 was almost the same in the whole permeation time. While in Fig. 5(a),although the amount ofM4HB andM2HB in the receiving chamber was nearly the same in the first 60 min,a larger difference appeared in the later 300 min and the maximum permeation separation factor ap could reach 3.122 (Table 5). These data indicated that the imprinted membranes did generate specific penetrating path for M4HB to pass and thus enhanced the perm- selectivity of themembrane.Moreover,the amount ofM4HB in the receiving chamber of MIOPM4 was much higher than that of NIOPM4 at the same time as shown in Table 5,which demonstrated that the membrane after imprinting significantly affected the transport amount of M4HB for the imprinted membrane.

|
Download:
|
| Fig. 5.Time-permeation curves of M4HB and M2HB through the MIOPM4 (a) and NIOPM4 (b) (feed concentration = 100 mg L-1 ). | |
| Table 5 Average permeation amount and separation factor for MIOPM4 and NIOPM4 (Operation Time = 380min). |
In our studies,the molecularly imprinted open porous membranes (MIOPMs) with interconnecting pores were prepared by combining the Pickering W/O HIPEs templating method with molecular imprinted technology. The obtained MIOPMs possessed the following interesting properties: (1) Incorporation of 2-EHA in the preparing process would increase the flexibility of the membranes without decreasing the adsorption capacity of M4HB. (2) The adsorption equilibrium and kinetic data matched Langmuir-Freundlich isotherm model and a pseudo-second-order kinetic model,respectively. (3) The MIOPMs had specific permeability for M4HB. These researching findings could be valuable for the recognition and detection of target molecules in the field of membrane separation.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Nos. 21406085,21207051).
| [1] | N.R. Cameron, D.C. Sherrington, High internal phase emulsions (HIPEs)—structure, properties and use in polymer preparation, Adv. Polym. Sci. 126 (1996) 163-214. |
| [2] | N. Brun, S. Ungureanu, H. Deleuze, R. Backov, Hybrid foams, colloids and beyond: from design to applications, Chem. Soc. Rev. 40 (2011) 771-788. |
| [3] | I. Pulko, P. Krajnc, Open cellular reactive porous membranes from high internal phase emulsions, Chem. Commun. 37 (2008) 4481-4483. |
| [4] | M.C. Hermant, M. Verhulst, A.V. Kyrylyuk, B. Klumperman, C.E. Koning, The incorporation of single-walled carbon nanotubes into polymerized high internal phase emulsions to create conductive foams with a low percolation threshold, Compos. Sci. Technol. 69 (2009) 656-662. |
| [5] | H.P. Gao, Y.X. Peng, J.M. Pan, et al., Synthesis and evaluation of macroporous polymerized solid acid derived from Pickering HIPEs for catalyzing cellulose into 5-hydroxymethylfurfural in an ionic liquid, RSC Adv. 4 (2014) 43029-43038. |
| [6] | E.H. Mert, M.A. Kaya, H. Yıldırım, Preparation and characterization of polyester-glycidyl methacrylate polyHIPE monoliths to use in heavy metal removal, Des. Monomers Polym. 15 (2012) 113-126. |
| [7] | C.H. Wang, X.X. Ma, C. Wang, Q.H. Wu, Z. Wang, Poly(vinylidene fluoride) membrane based thin film microextraction for enrichment of benzoylurea insecticides from water samples followed by their determination with HPLC, Chin. Chem. Lett. 25 (2014) 1625-1629. |
| [8] | Z.L. Yang, J.L. Li, C.L. Zhang, Y.F. Lu, Z.Z. Yang, Two-dimensional mesoporous materials: from fragile coatings to flexible membranes, Chin. Chem. Lett. 24 (2013) 89-92. |
| [9] | Y.J. Lu, J.H. Jia, The effect of complexing agent on crystal growth, structure and properties of nanostructured Cu2-xS thin films, Chin. Chem. Lett. 25 (2014) 1473-1478. |
| [10] | J.S. Park, E. Ruckenstein, Selective permeation through hydrophobic-hydrophilic membranes, J. Appl. Polym. Sci. 38 (1989) 453-461. |
| [11] | E. Ruckenstein, J.S. Park, The separation of water-ethanol mixtures by pervaporation through hydrophilic-hydrophobic composite membranes, J. Appl. Polym. Sci. 40 (1990) 213-220. |
| [12] | P. Krajnc, N. Leber, D. Štefanec, S. Kontrec, A. Podgornik, Preparation and characterisation of poly(high internal phase emulsion) methacrylate monoliths and their application as separation media, J. Chromatogr. A 1065 (2005) 69-73. |
| [13] | I. Pulko, V. Smrekar, A. Podgornik, P. Krajnc, Emulsion templated open porous membranes for protein purification, J. Chromatogr. A 1218 (2011) 2396-2401. |
| [14] | J. Lee, S. Bernard, X.C. Liu, Nanostructured biomimetic catalysts for asymmetric hydrogenation of enamides using molecular imprinting technology, React. Funct. Polym. 69 (2009) 650-654. |
| [15] | M.J. Whitcombe, N. Kirsch, I.A. Nicholls, Molecular imprinting science and technology: a survey of the literature for the years 2004-2011, J. Mol. Recognit. 27 (2014) 297-401. |
| [16] | K. Haupt, Molecularly imprinted polymers: the next generation, Anal. Chem. 75 (2003) 376A-383A. |
| [17] | S.G. Dmitrienko, V.V. Irkha, V.V. Apyari, E.V. Klokova, Y.A. Zolotov, Recognition of hydroxybenzoic acids and their esters by molecularly imprinted polymers, Mendeleev Commun. 18 (2008) 315-317. |
| [18] | M.S. da Silva, R. Viveiros, V.D.B. Bonifá cio, A. Aguiar-Ricardo, T. Casimiro, Supercritical fluid technology as a new strategy for the development of semi-covalent molecularly imprinted materials, RSC Adv. 2 (2012) 5075-5079. |
| [19] | A. Lourenço, R. Viveiros, A. Moro, et al., Supercritical CO2-assisted synthesis of an ultrasensitive amphibious quantum dot-molecularly imprinted sensor, RSC Adv. 4 (2014) 63338-63341. |
| [20] | Y.L. Wu, Y.S. Yan, J.M. Pan, et al., Fabrication and evaluation of molecularly imprinted regenerated cellulose composite membranes via atom transfer radical polymerization, Chin. Chem. Lett. 25 (2014) 273-278. |
| [21] | M. Fang, F. Lei, J. Zhou, Y.N. Wu, Z.Y. Gong, Rapid, simple and selective determination of 2,4-dinitrophenol by molecularly imprinted spin column extraction coupled with fluorescence detection, Chin. Chem. Lett. 25 (2014) 1492-1494. |
| [22] | J.M. Pan, Q. Qu, J. Cao, et al., Molecularly imprinted polymer foams with welldefined open-cell structure derived from Pickering HIPEs and their enhanced recognition of l-cyhalothrin, Chem. Eng. J. 253 (2014) 138-147. |
| [23] | M.G. Soni, S.L. Taylor, N.A. Greenberg, G.A. Burdock, Evaluation of the health aspects of methyl paraben: a review of the published literature, Food Chem. Toxicol. 40 (2002) 1335-1373. |
| [24] | O. Handa, S. Kokura, S. Adachi, et al., Methylparaben potentiates UV-induced damage of skin keratinocytes, Toxicology 227 (2006) 62-72. |
| [25] | Y. Okamoto, T. Hayashi, S. Matsunami, K. Ueda, N. Kojima, Combined activation of methyl paraben by light irradiation and esterase metabolism toward oxidative DNA damage, Chem. Res. Toxicol. 21 (2008) 1594-1599. |
| [26] | K. Kannathasan, A. Senthilkumar, V. Venkatesalu, Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn, Acta Trop. 120 (2011) 115-118. |
| [27] | S.W. Zou, Y. Yang, H. Liu, C.Y. Wang, Synergistic stabilization and tunable structures of Pickering high internal phase emulsions by nanoparticles and surfactants, Colloid Surf. A 436 (2013) 1-9. |
| [28] | Y.L. Wu, M.J. Meng, X.L. Liu, et al., Efficient one-pot synthesis of artemisininimprinted membrane by direct surface-initiated AGET-ATRP, Sep. Purif. Technol. 131 (2014) 117-125. |
| [29] | S.J. Allen, G. Mckay, J.F. Porter, Adsorption isotherm models for basic dye adsorption by peat in single and binary component systems, J. Colloid Interface Sci. 280 (2004) 322-333. |
| [30] | M. Mazzotti, Equilibrium theory based design of simulated moving bed processes for a generalized Langmuir isotherm, J. Chromatogr. A 1126 (2006) 311-322. |
| [31] | Y.S. Ho, G. McKay, The sorption of lead (II) ions on peat, Water Res. 33 (1999) 578-584. |
| [32] | Y.S. Ho, G. McKay, Pseudo-second order model for sorption processes, Process Biochem. 34 (1999) 451-465. |