b Department of Bioengineering and Environment Science, Changsha University, Changsha 410003, China
The sensitive and convenient detection of single nucleotide polymorphisms (SNPs) is important in biological studies,drug development and clinical diagnostics [1, 2]. A series of SNP detection systems were developed,such as polymerase chain reaction amplification [3],gel electrophoresis [4],rolling circle amplification [5, 6],strand-displacement of polymerase [7, 8, 9], locked nucleic acid (LNA)-based northern blot [10],specific enzymatic cleavage [11] and so on. However,these methods usually are enzyme-assisted complicated strategies [12]. It remains necessary and meaningful to develop a simple and enzyme-free strategy for SNPs detection at normal temperature.
The strand displacement reaction (SDR) can be initiated by a strand hybridization with the complementary single stranded overhang domains (known as toeholds) of prehybridized strands and progresses (toehold-SDR) through a branch migration process [13, 14, 15]. Toehold-SDR can take place without an enzyme at room temperature,and the kinetic rate can be controlled by adjusting the length and sequence composition of the toehold,which is used widely in analyzing SNPs as its enzyme-free,robustness,specificity and isothermality [16, 17, 18]. Li et al. combined gold nanoparticle with fluorescence anisotropy for the assay of SNP with the detection limit of 0.95 nmol/L based on toehold-mediated SDR [17] and Wang et al. combined quartz crystal microbalance and toehold-SDR for the detection of SNP with 0.3 nmol/L limit [18]. Until now,there has been no consensus for the best enzyme-free SNP detection method. There is a continuous need for new SNP detection methods to meet specific needs and situations.
In thiswork,we developed a novelmicrobead-assisted toehold- SDR biosensor to detect SNP for the first time. The biosensor consists of a pre-hybridized strand formed by a reporter probe and a capture probe. In the presence of a mutant sequence,there is no toehold-mediated strand displacement and the reporter probe cannot be released from the pre-hybridized strand. When adding microbeads,the fluorescent pre-hybridized strand is captured by microbeads,resulting in that the solution is no obvious fluores- cence,whilemicrobeads appear significant fluorescence. However, in the presence of a matched target,the strand displacement is effectively initiated and the reporter probe is released from pre- hybridized strand. After adding microbeads,the solution produces bright fluorescence,while microbeads have no obvious fluores- cence signal. The target sequence is discriminated perfectly from the single-base mutant sequences.
2. Experimental 2.1. MaterialsAll synthesized and HPLC-purified sequences of oligos as depicted in Table 1 were commercially ordered from TaKaRa Bio Inc. (Dalian,China). The capture probe strand was 5' -biotin labeled sequence including 4-nt poly(T) spacer,6-nt toehold region,and 20-nt template sequence. The single-base mismatched sequences were designed such that the mismatched site located in the sixth base of toehold region from 5' -end. The reporter probe was a 5' FAM-labeled sequence complementary to the template strand of the capture probe. Streptavidin-coated microbeads were pur- chased from Sigma-Aldrich (MO,USA). All other reagents were of analytical grade. Deionizedwaterwas obtained fromthe Nanopure InfinityTM ultrapure water system (Barnstead/Thermolyne Corp., Dubuque,IA,USA).
| Table 1 Sequence of oligos in this study.a. |
The proposed method employed microbeads to capture biotin- label strands to produce signal difference through biotin-streptavidin interaction. Firstly,microbeads were washed twice in PBS solution as described before [19]. The detection process involved the following twomajor steps: (1) adding a target into the pre-hybridized strand between a capture probe and a reporter probe to perform the toehold-SDR; (2) capturing biotin-label strands using streptavidin-coated microbeads for the effective detection. Unless specified,the reaction buffer was used contain- ing 200 nmol/L capture probe,200 nmol/L reporter probe, 20 mmol/L Tris-HCl pH 7.4,and 15 mmol/L MgCl2. Target II was added into the reaction buffer with the final concentration as indicated,which mixed and incubated for 30 min to perform a toehold-SDR. Then,microbeads were added to the mixture and incubated for 50 min with end-to-end shaking to capture biotin- label strands. Then,samples were kept quietly for 3 min and the fluorescence for the supernatant solution and the bottom of microbeads were detected,respectively. All procedures were performed at normal temperature.
2.3. Fluorescence measurement of the solutionThe fluorescence intensities were recorded using F-7000 fluorescence spectrophotometer (Hitachi,Japan). The fluorescent spectra were measured using the spectrofluorophotometer. The excitation wavelength was 490 nm,and the spectra were recorded between 500 and 600 nm. The fluorescence emission intensitywas measured at 520 nm.
2.4. Fluorescent detection of microbeadsFluorescence of the microbead was achieved with a fluores- cence microscope (Leica DM IRB,Leica Corp.,Germany) equipped with a CCD camera (Leica DC 300F) and the corresponding quantitative values were obtained by calculating the gray scale of fluorescent particles with the software Image J [19, 20].
3. Results and discussion 3.1. Assay principleThe design of our microbead-assisted toehold-mediated strand displacement biosensor is shown in Fig. 1. The biosensor consists of a pre-hybridized strand between a reporter probe and a capture probe. The reporter probe is a FAM-label single DNA. The capture probe is biotin-label sequence containing a 4-nt link sequence,6- nt toehold domain,and 20-nt template sequence,in which the sequence of both the toehold and template is exactly complemen- tary to the target,the template sequence is exactly complementary to the reporter probe,and the poly (T) link sequence is not complementary to any sequence. The microbead used is coated by streptavidin. In the presence of a target,the target is introduced as an invading DNA to initiate the strand displacement reaction by hybridization with the toehold domain of the capture probe, resulting in the release of FAM-label reporter probe from the pre- hybridized strand,and at the same time forming newhybridization strand between the capture probe and the target.When adding the streptavidin-coated microbeads,the no-fluorescence new hybrid- ization strand is captured,which results in that microbeads have no obvious fluorescence signal,while the solution produces bright fluorescence (Fig. 1A). However,for the single-base mismatch sequence,the strand displacement process is restrained because the mismatch suppresses the hybridization between the capture probe and single-base mutant sequences [17, 18]. The reporter probe cannot effectively release from the pre-hybridized strand. When addingmicrobeads,the pre-hybridized strand is captured by the microbead. This results in that microbeads produce bright fluorescence,while there is no obvious fluorescence in the solution (Fig. 1B). Following this design strategy,the target sequence is discriminated from the single-base mutant sequences.

|
Download:
|
| Fig. 1.The design of the microbead-assisted toehold-SDR for point mutations. | |
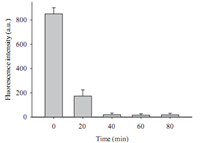
It is shown clearly in Fig. 1 that this proposed method employs microbeads to capture biotin-label strands through biotin- streptavidin interaction to perform the detection. The capture efficiency ofmicrobeads is very important to establish themethod. Therefore,some experiments were performed to investigate the capture efficiency of the microbeads at different capturing time using the pre-hybridized strand between a reporter probe and a capture probe as capturing object. It is well known that there will be no fluorescence in the supernatant solution after efficient microbead-capture. Fig. 2 was the fluorescence intensity of the solution for different microbead-capturing times. It showed that the solution still kept obvious fluorescence during at 20 min capturing time; however,there was weak fluorescence at capturing times for 40,60,and 80 min,respectively. This indicated that the microbeads captured effectively these pre-hybridization from the solution in range from 40 to 80 min,which resulted in there was no obvious fluorescence signal in the solution. This indicated that capturing time range from40 to 80 minwas enough. To ensure efficient capturing time,50 min was chosen as the capturing time in following experiments.
|
Download:
|
| Fig. 2. The fluorescent signal of the solution after adding microbeads to capture FAM-labeled pre-hybridization strands. | |
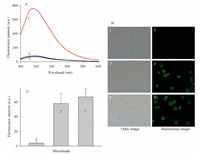
We verified the detection capability of this sensing system by the fluorescence of the solution (Fig. 3A) and microbeads (Fig. 3B) using three samples. Sample 1 added a matched target in the biosensor,sample 2 added a single-base mutant sequence,and sample 3 added a random sequence. It was found in the sample 1 that there was very significant fluorescence signal in the solution (curve 1 in Fig. 3A). However,microbeads had no obvious fluorescence (Image 1 in Fig. 3B). The reason maybe was that the target triggered the strand displacement,resulting in that the reporter probe was released frompre-hybridized strand. However, for themutant sequence (sample 2). Itwas found that therewas no obvious fluorescence signal in the solution (curve 2 in Fig. 3A), while microbeads produced significant fluorescence (Image 2 in Fig. 3B). This maybe results from that mutant sequence cannot trigger strand displacement reaction,so the reporter probe cannot be released from the pre-hybridized strand. When adding microbeads,the fluorescent pre-hybridized strand is captured by the microbead. The similar results were found for the random DNA (curve 3 and Image 3 in Fig. 3). These results clearly indicated the detection capability of the biosensor using the solution- fluorescence strategy and microbead-signal strategy. In the following experiments,we used the solution-fluorescence strategy to realize the detection considering few detection procedures.
|
Download:
|
| Fig. 3. The fluorescence responses of the biosensor with three samples. Samples 1-3 were added 200 nmol/L target II,mutant T and random DNA at reaction buffer, respectively. (A) The spectra of the solution of three samples. (B) The images of microbeads fromthree samples. (C) The corresponding fluorescence strength column blots of Image 1-3. | |
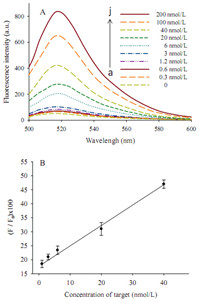
Under the optimized conditions,the relationship between the fluorescence response of the solution and the concentration of the target was investigated (Fig. 4A). It was shown that the fluorescence signals increased remarkably with the increase of the target concentration from 0.3 nmol/L to 200 nmol/L. It indicated that the number of the release of reporter probe from the pre-hybridized strand triggered by the target increased with the increase of the target. Moreover,this biosensor showed the linear relationship in the range of 1-40 nmol/L (R = 0.9953) and the detection limit of 0.3 nmol/L calculated by the triple signal-to- noise method,about 30 fmol with a detection volume of 100 mL. The limit of detection of the proposed method with enzyme-free, simplification,and sensitivity was comparable to that of existing toehold-SDR methods.
|
Download:
|
| Fig. 4. The sensitivity of the proposed biosensor. (A) Effect of different target concentrations on fluorescence emission spectra. (B) Linear relationship between the fluorescence intensity and the concentration of target. | |
Then,we used various oligos as the matched target,different mutant sequences and random DNAs to investigate the specificity of the biosensor (Fig. 5). The target is complementary to the capture probe. The mutant DNA is a single base different from the matched target (Table 1). Itwas shown that the fluorescence signal was significantly larger in the solution containing the matched target than that of other DNAs containing mutant and random DNAs,indicating a high specificity of the detection system. The high specificity may be attributed to the mismatch between the mutant sequence and the capture probe greatly prevents the impending strand displacement.

|
Download:
|
| Fig. 5. Specificity response of the proposed biosensor to 200 nmol/Lmatched target II,mismatchedmutant T,mismatchedmutant G,mismatchedmutant C and random DNA. | |
In this work,we proposed an enzyme-free microbead-assisted strategy for SNP detection with simplicity,sensitivity and selectivity at normal temperature. This assay design showed good differentiation between perfectly matched and single-base mismatched sequences to avoid effectively false-positive results. The proposed sensing strategy not only extends the application of the toehold-mediated strand displacement and microbead tech- nique,but also provides a simple and universal strategy for SNP detection through easily altering the sequences according to the sequences around target SNPs,indicating that our biosensor had the potential for biological sample application.
AcknowledgmentsThis work is supported by National Natural Science Foundation of China (No. 21275043) and National Basic Research Program of China under Grants (No. 2009CB421601).
| [1] | L. Kruglyak, D.A. Nickerson, Variation is the spice of life, Nat. Genet. 27 (2001) 234-236. |
| [2] | D.J. Eastburn, A. Sciambi, A.R. Abate, Picoinjection enables digital detection of RNA with droplet RT-PCR, PLoS One 8 (2013) e62961. |
| [3] | A. Klindworth, E. Pruesse, T. Schweer, et al., Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies, Nucleic Acids Res. 41 (2013) e1. |
| [4] | H.L. Cheng, S.S. Chiou, Y.M. Liao, Y.L. Chen, S.M. Wu, Genotyping of single nucleotide polymorphism in γ-glutamyl hydrolase gene by capillary electrophoresis, Electrophoresis 32 (2011) 2021-2027. |
| [5] | T. Murakami, J. Sumaoka, M. Komiyama, Sensitive isothermal detection of nucleicacid sequence by primer generation-rolling circle amplification, Nucleic Acids Res. 37 (2009) e19. |
| [6] | Y.T. Zhou, Q. Huang, J.M. Gao, et al., A dumbbell probe-mediated rolling circle amplification strategy for highly sensitive microRNA detection, Nucleic Acids Res. 38 (2010) e156. |
| [7] | F. Xuan, X.T. Luo, I.M. Hsing, Sensitive immobilization-free electrochemical DNA sensor based on isothermal circular strand displacement polymerization reaction, Biosens. Bioelectron. 35 (2012) 230-234. |
| [8] | H.Y. Su, X.C. Meng, Q.P. Guo, et al., Label-free DNA sensor with PCR-like sensitivity based on background reduction and target-triggered polymerization amplification, Biosens. Bioelectron. 52 (2014) 417-421. |
| [9] | L.I. Ward, S.J. Harper, Loop-mediated isothermal amplification for the detection of plant pathogens, Methods Mol. Biol. 862 (2012) 161-170. |
| [10] | A. Valoczi, C. Hornyik, N. Varga, et al., Sensitive and specific detection of micro-RNAs by northern blot analysis using LNA-modified oligonucleotide probes, Nucleic Acids Res. 32 (2004) e175. |
| [11] | D.J. Caruana, A. Heller, Enzyme-amplified amperometric detection of hybridization and of a single base pair mutation in an 18-base oligonucleotide on a 7-mmdiameter microelectrode, J. Am. Chem. Soc. 121 (1999) 769-774. |
| [12] | K. Knez, D. Spasic, K.P.F. Janssen, J. Lammertyn, Emerging technologies for hybridization based single nucleotide polymorphism detection, Analyst 139 (2014) 353-370. |
| [13] | Y. Xiao, B.D. Piorek, K.W. Plaxco, A.J. Heeger, A reagentless signal-on architecture for electronic, aptamer-based sensors via target-induced strand displacement, J. Am. Chem. Soc. 127 (2005) 17990-17991. |
| [14] | A.J. Genot, D.Y. Zhang, J. Bath, A.J. Turberfield, Remote toehold: amechanism for flexible control of DNA hybridization kinetics, J. Am. Chem. Soc. 133 (2011) 2177-2182. |
| [15] | B. Yurke, A.P. Mills Jr., Using DNA to power nanostructures, Genet. Program Evolv. Mach. 4 (2003) 111-122. |
| [16] | Z. Zhang, D.D. Zeng, H.W. Ma, et al., A DNA-origami chip platform for label-free SNP genotyping using toehold-mediated strand displacement, Small 6 (2010) 1854-1858. |
| [17] | X.Y. Wang, M.J. Zou, H.D. Huang, et al., Gold nanoparticle enhanced fluorescence anisotropy for the assay of single nucleotide polymorphisms (SNPs) based on toehold-mediated strand-displacement reaction, Biosens. Bioelectron. 41 (2013) 569-575. |
| [18] | D.Z. Wang, W. Tang, X.J. Wu, et al., Highly selective detection of singlenucleotide polymorphisms using a quartz crystal microbalance biosensor based on the toehold-mediated strand displacement reaction, Anal. Chem. 84 (2012) 7008-7014. |
| [19] | X.X. Meng, X.H. Yang, K.M. Wang, et al., Direct fluorescence detection of point mutations in human genomic DNA using microbead-based ligase chain reaction, Talanta 80 (2010) 1725-1729. |
| [20] | L.J. Zhou, K.M. Wang, W.H. Tan, et al., Quantitative intracellular molecular profiling using a one-dimensional flow system, Anal. Chem. 78 (2006) 6246-6251. |





