b Department of Chemistry, Xixi Campus, Zhejiang University, Hangzhou 310028, China;
c College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China
Over the past three decades,ion chromatography (IC) has blossomed into an excellent tool for the analysis of charged species such as inorganic ions and small ionizable molecules. With the same principles as HPLC,the stationary phase plays a very important role in the process of ion chromatographic separation. Therefore,developing a new stationary phase is extremely beneficial (necessary) and meaningful [1, 2]. In IC,the stationary phase mainly consists of silica-based and polymer-based exchan- gers [3, 4]. Silica-based exchangers [5, 6, 7] are rigid,but have a limitation in their operating pH range because the silica backbone is unstable at pH < 2 and pH > 8. Polymer-based columns aremore commonly used in IC due to tolerance to high pH eluents. Polystyrene-divinylbenzene (PS-DVB) copolymer anion exchan- gers [3, 8] are commonly used for anion separation by IC. The methods of preparation of stationary phases includes surface- grafted [9, 10, 11, 12],surface-embedded and surface-agglomerated [3, 13, 14, 15, 16]. Agglomerated anion exchange phases consist of a polymer core bearing sulfonic acid groups with an agglomerated layer of nanometer-sized polymer particles (latex) functionalized with alkyl quaternary ammoniumgroups [17, 18]. This approach of stationary phase preparation provides a number of significant advantages including compatibility with pH 0-14 of the mobile phase when the particles are prepared with styrene based monomers and excellent long-term chemical stability in order to provide long-term consistency of stationary phase selectivity [3]. However,the polymeric phases have lower thermal and mechanical stability,and lower efficiencies compared with inorganic oxides such as silica and zirconia [19]. Therefore,the development of new stationary phases continues to be one of the most challenging research goals in IC.
Alkyl quaternary ammonium groups are commonly used as modifiers of a cation-exchange chromatographic stationary phase. Poly-epichlorohydrin-dimethylamine (PEPI-DMA),which is an effectively water-soluble cationic polyelectrolyte with amidocya- nogen and ammonium ion,has been applied in the treatment of printing and dyeing wastewater [20, 21, 22, 23, 24]. In this paper,a polystyrene-divinylbenzene stationary phase agglomerated with PEPI-DMA for IC was synthesized and studied.
Iodine is an essential element for human beings. The lack of iodine leads to iodine deficiency disorder (IDD),while excessive dietary intake of iodine can result in serious pathological problems [25, 26]. A number of analytical methods have been developed for the determination of iodide in seawater [27, 28], povidone iodine solution[26],urine [29],edible seaweed[25],and other samples [30, 31]. Also,the determination of iodide directly, or indirectly,has been accomplished by catalytic spectrophotom- etry [25],IC with columns and column-switching technique [26], HPLC with pulsed amperometric detector [29],electrostatic ion chromatography [28],polymeric membrane sensors [30],flow injection [32],capillary electrophoresis [27],and gas chromatog-raphy-mass spectrometry after derivatization [33]. However,the pretreatment processes required and the analytical processes of samples are complex in themethods above. Themethod described in this paper is based on a new homemade stationary phase which was successfully applied to the determination of iodide in povidone iodine solutions and kelp samples directly and conveniently.
2. Experimental 2.1. EquipmentThe chromatographic system consisted of an DX-120 ion chromatograph (DIONEX,Sunnyvale,CA) with a dual-piston serial pump,a Rheodyne (Cotati,CA,USA) six-port valve,a 25-mL sampling loop,a conductivity detector or an ED40 electrochemical detector equipped with a platinum working electrode and an Ag/ AgCl reference electrode. Chromeleon 6.8 chromatography data management software (Thermo Scientific,Sunnyvale,CA,USA) was used for system control and data processing. The voltage parameter for the DC amperometric detector was 0.90 V. The eluent contained 5 mmol/L HNO3 and 15 mmol/L NaNO3 with a flow rate 1.0 mL/min. The packing system consisted of a nitrogen cylinder,a pneumatic pump,a slurry reservoir,jointing units,and stainless-steel analytical columns.
2.2. ReagentsThe 1,2-diaminoethane was purchased from Aladdin Chemical Co.,Ltd. (Shanghai,China). Dimethylamine (DMA,33% in H2O,v/v), epichlorohydrin (EP),ethanol,nitric acid,sodium nitrate,potassi- um iodide,sulfuric acid,glacial acetic acid and dichloromethane were purchased fromHuipu Chemical Reagent Co.,Ltd. (Hangzhou, China).
Polystyrene-divinylbenzene (PS-DVB) substrate beads were synthesized according to the literature [24]. A water purification system (Millipore,Milford,MA,USA) was used to further deionize distilled water for all eluents and sample mixtures. Standard solutions of F-,Cl -,Br -,NO2 -,NO3 -,SO4 2-,S2O3 2-,I - and SCN- ions were prepared by diluting the stock solutions to required concentration just prior to use. Other chemicals were used as received.
2.3. Sample treatmentThe sample of povidone iodine solution was produced by dissolving the proper amount of povidone iodine solution in 100 mL deionized water,and filtered through a 0.22 mm mem- brane filter and SPE-C18 column (Tianjin Fuji Science & Technology Co. Ltd.,Tianjin,China) before injection.
The sample of kelp was randomly bought at a local market. The treatment process was as follows: Firstly,the sample,after removal of sediment,was chopped up and dried at 60 8C and then the sample was milled and accurately weighed in 50 mL deionized water,then subjected to ultrasonification for 1 h. Finally the samplewasmixed thoroughly before centrifuging for 20 min at 12,000 rpm,and the supernatant solution was filtered through a 0.22 mmmembrane and SPE-C18 column before injection. The first 1 mL of eluate was discarded and the following solution was collected for further analysis.
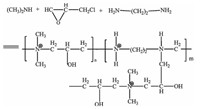
2.4. Preparation of anion exchange column 2.4.1. Synthesis of PEPI-DMAThe synthesis procedure of PEPI-DMA was according to the literature [34] and the reaction equation is shown in Fig. 1. Specifically,the polymerization was carried out in an ethanol solution as follows: Dimethylamine (13.66 g) was first added to a 250 mL glass reactor equipped with a temperature controller at 0 oC initially with a magnetic stirrer,then,cold epichlorohydrin (13.88 g) at a dimethylamine to epichlorohydrin molar ratio (1:1.5) was added dropwise into the reactor with constant stirring to form oligomers. Next,1,2-diaminoethane at a chosen weight percentage (3%) of the mixture was added dropwise into the reactor under stirring and then,the reactive temperature was raised slowly to 70 8C. The polyamine polymers were finally obtained after 7 h reaction.
|
Download:
|
| Fig. 1.The reaction equation of PEPI-DMA. | |
The synthesis procedure of PS-DVB and sulfonated PS-DVB has been reported previously [26]. An aqueous solution of PEPI-DMA was added to another aqueous suspension of sulfonated PS-DVB, the reaction mixture was stirred at 70 8C for 2 h.
2.4.3. Column packing procedureThe columnwas packed by a slurry packing technique. A 3.0 g of slurry of PEPI-DMA agglomerated PS-DVB beads was dispersed into 60 mL of deionized water and sonicated in an ultrasonicator for 10 min and then pressed into an empty column (stn stl, 200 mm - 4.0 mm,id),with deionized water as packing solvent at a working pressure of 3000 psi. The volume of packing solvent passing through the column should be at least 400 mL. The resulting anion exchange column was washed with 30 mmol/L NaOH solution for at least 12 h before use in the IC instrument.
3. Results and discussion 3.1. Characterizations of the PS-DVB-based stationary phaseThe characteristics of the PS-DVB-based stationary phase with PEPI-DMAwere examined by scanning electronmicroscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and elemental analysis. The porous properties of the particles were characterized by the chemical adsorption and desorption method and calculated by Brunauer-Emmett-Teller (BET) method [10]. The capacity of the column was determined using the breakthrough curvemethod [26].
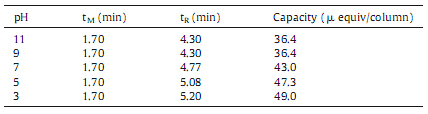
The chromatographic conditions: 10 mmol/L of sodium nitrate solution was used as eluent at a flow rate of 1.4 mL/min. The capacities were determined by direct UV absorbance (l = 215 nm) in different pH which are shown in Table 1. The capacity increased as pH decreased when the pH value was lower than nine. This is attributed to the tertiary amino groups in the PS-DVB-based stationary phase with PEPI-DMA,specifically,more tertiary amino groups changed to quaternary amino groups as the pH decreased, therefore the higher the capacity of the particles.
| Table 1 The capacity in different pH. |
The FTIR spectra of raw PS-DVB particles,sulfonated PS-DVB particles and PEPI-DMA agglomerated PS-DVB particles are shown in Fig. 2. Compared to raw PS-DVB particles,new absorption bands at 1176 cm-1 were observed in the case of sulfonated PS-DVB particles due to a stretching vibration of S5 5O of the -SO3H group. This result indicated the incorporation of sulfonic acid groups on (to) the PS-DVB particles after the treatment of concentrated sulfuric acid. The peak at 1176 cm-1 was found to disappear after anchoring the PEPI-DMA on the sulfonated PS-DVB particleswhich may be due to formation of ionic bonding between sulfonic acid groups and PEPI-DMA weakening the absorption of -SO3H.Additionally,the peak at 1060 cm-1 was remarkably enhanced in the PEPI-DMA agglomerated PS-DVB particles,which is characterized by the stretching vibration of C-N group.

|
Download:
|
| Fig. 2. FTIR spectra of raw PS-DVB particles,sulfonated PS-DVB particles and PEPI-DMA agglomerated PS-DVB particles. | |
SEMwas performed to observe themorphologies of the rawPS- DVB particles and sulfonated PS-DVB particles and PEPI-DMA agglomerated PS-DVB particles in Fig. 3. The images of rawPS-DVB particles showed relatively clean and smooth surfaces for the as-prepared PS-DVB beads. Compared with the SEM image of the raw PS-DVB particles,it was seen clearly that the sulfonated PS-DVB particles were not destroyed and the surfaces of the agglomerated PS-DVB beads were immobilized with polymer- epichlorohydrin-dimethylamine (PEPI-DMA).
|
Download:
|
| Fig. 3.SEM of raw styrene-divinylbenzene particles (A),sulfonated PS-DVB particles (B) and PEPI-DMA agglomerated PS-DVB particles (C). | |
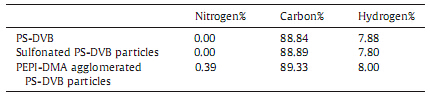
The results of elemental analysis of the particles are given in Table 2. The nitrogen content of the coated particles was obviously higher than that of rawPS-DVB particles,which confirmed that the PEPI-DMA was successfully immobilized onto the surface of the raw PS-DVB particles.
| Table 2 Elemental analysis results of the particles. |
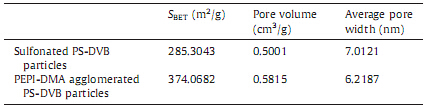
The porous properties of the particles are shown in Table 3. After agglomerating PEPI-DMAs,the surface areas and pore volumes of PS-DVB particles increased,while the average pore widths decreased. This may be due to the addition of PEPI-DMAs that coated the surface of particles and lead to increasing surface areas and reducing average pore widths.
| Table 3 Porosity characteristics of the particles. |
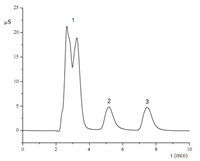
The chromatographic evaluation of the PS-DVB/PEPI-DMA stationary phase was performed using a test mixture of inorganic anions composed of F-,Cl -,NO2 -,Br -,NO3 -,SO4 2-,S2O3 2-,I - and SCN-. A 30 mmol/L NaOH solution was used as eluent with the flow rate of 1.0 mL/min with conductivity detection. The result is shown in Fig. 4. The iodide ion was well separated from other ions as above-mentioned.
|
Download:
|
| Fig. 4. The chromatogramof a standard solutionmixture. 1: F-(2mg/L),Cl -(4mg/L), NO2 -(10 mg/L),Br -(20 mg/L),NO3 -(20 mg/L),SO4 2-(20 mg/L),S2O3 2-(20 mg/L); 2:I-(20 mg/L); 3: SCN-(20mg/L). | |
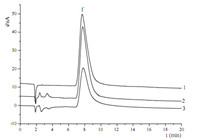
Due to the lowsensitivity of iodidewith conductivity detection, the new PS-DVB/PEPI-DMA stationary phase was utilized to the determine iodide content of two samples with DC amperometric detection and the HNO3-NaNO3 complex solution used as eluent and a flow rate of 1 mL/min. A platinum working electrode and an Ag/AgCl reference electrode and voltage 0.90 V were chosen based on the highest S/N (Signal to Noise ratio). Different acid concentrations and eluent concentrations were also optimized. The eluent solution containing 5 mmol/L of HNO3 and 15 mmol/L of NaNO3 was chosen finally. Under the optimized conditions,the relative standard deviation (RSD) of the retention time,peak areas and peak heights of 5 mg/L were all less than 1% (n = 6).
The limit of detection (S/N ratio 3:1) was 0.05 mg/L. A six-point calibration curve,ranging from 0.2 mg/L to 50 mg/L,yielded the linear equation I = 19.5437c (mg/L) + 0.8237 with coefficient correlation 0.9990 for peak heights.
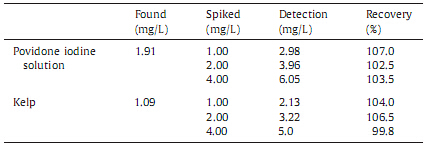
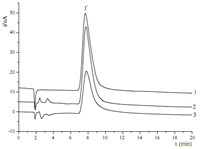
The optimized method was evaluated by analyzing the presence of iodide in povidone iodine solutions and kelp samples. The povidone iodine solution was diluted 10,000 folds,while the kelp samples were diluted 4000 folds. After treatment and filtration,the samples were injected directly. The results and chromatograms are shown in Table 4 and Fig. 5.
| Table 4 Results and recoveries of iodide ion from sample of povidone iodine solution and kelp sample (n = 6). |
|
Download:
|
| Fig. 5. Ion chromatograms of (1) standard solution (2 mg/L),(2) povidone iodine solution and (3) kelp sample. | |
Upon calculation,the concentrations of iodide anion are 19.10 g/L in the sample of povidone iodine solution and 4.36 mg/kg in the kelp sample,respectively.
4. ConclusionThe new stationary phase based on PS-DVB resin using PEPI- DMA as modifier in IC was prepared and characterized. The stationary phase was characterized by Fourier transform infrared spectroscopy (FTIR),scanning electron microscopy (SEM),and elemental analysis. The porous properties of the particles were characterized by the chemical adsorption and desorption method and calculated by Brunauer-Emmett-Teller (BET) method. The results showed that PEPI-DMA was successfully bonded (coated) onto the surface of PS-DVB particles. And the capacity of the column was determined using the breakthrough curve method. Finally,the chromatographic evaluation of the novel homemade column was performed by chromatographing using various anions with conductivity detection. The iodide ion was well separated from other ions studied.
The fabricated column provedwell suited for the determination of iodide ion in povidone iodine solutions and kelp samples with DC amperometric detection. The iodide ion can be determined within 10 min. Under the optimum conditions,the linear range of the method was 0.2-50 mg/L for iodide ion with a correlation coefficient of 0.9990,the RSDs were all less than 1%,and the column showed excellent stability for parameters including retention times,peak areas and peak heights. The recoveries of the method for all sample determinations were in the range of 99.8%-107.0%. The analysis application of the homemade column in other areas need further study.
AcknowledgmentsThis research was financially supported by Natural Science Foundation of Zhejiang Province (Nos. Y4110532,LQ13B050001, LY12B05003); Professional Development Program for Visiting Scholars from Colleges and Universities in Zhejiang Province (No. FX2013148); and Major National Scientific Instrument and Equipment Development Special of China (No. 2012YQ09022903), Key Laboratory of Health Risk Appraisal for Trace Toxic Chemicals of Zhejiang Province.
| [1] | D. Gao, F.C. Tan, W.P. Wang, L.L. Wang, Resolution enhancement in hydrophobic interaction chromatography via electrostatic interactions, Chin. Chem. Lett. 24 (2013) 419-421. |
| [2] | Q.J. Peng, J.J. Yang, Preparation and chromatographic characteristics of a novel 2,6-dimethyl-β-CD bonded HPLC chiral stationary phase, Chin. Chem. Lett. 25 (2014) 1416-1418. |
| [3] | Z.P. Huang, H.W. Wu, F.L. Wang, et al., Polystyrene-divinylbenzene stationary phases agglomerated with quaternized multi-walled carbon nanotubes for anion exchange chromatography, J. Chromatogr. A 1294 (2013) 152-156. |
| [4] | L.M. Nair, R. Saari-Nordhaus, R.M. Montgomery, Applications of a new methacrylate-based anion stationary phase for the separation of inorganic anions, J. Chromatogr. A 789 (1997) 127-134. |
| [5] | A.V. Pirogov, O.V. Krokhin, M.M. Platonov, Y.I. Deryugina, O.A. Shpigun, Ionchromatographic selectivity of polyelectrolyte sorbents based on some aliphatic and aromatic ionenes, J. Chromatogr. A 884 (2000) 31-39. |
| [6] | A.V. Pirogov, M.M. Platonov, O.A. Shpigun, Polyelectrolyte sorbents based on aliphatic ionenes for ion chromatography, J. Chromatogr. A 850 (1999) 53-63. |
| [7] | O.V. Krokhin, A.D. Smolenkov, N.V. Svintsova, O.N. Obrezkov, O.A. Shpigun, Modified silica as a stationary phase for ion chromatography, J. Chromatogr. A 706 (1995) 93-98. |
| [8] | M.P. Raskop, A. Grimm, A. Seubert, Polystyrene immobilized ionenes as novel stationary phase for ion chromatography, Microchim. Acta 158 (2007) 85-94. |
| [9] | N.N. Wang, S.W. He, Y. Zhu, Low-level bromate analysis by ion chromatography on a polymethacrylate-based monolithic column followed by a post-column reaction, Eur. Food Res. Technol. 235 (2012) 685-692. |
| [10] | Y.Y. Zhong, W.F. Zhou, P.M. Zhang, Y. Zhu, Preparation, characterization, and analytical applications of a novel polymer stationary phase with embedded or grafted carbon fibers, Talanta 82 (2010) 1439-1447. |
| [11] | E. Unsal, T. Irmak, E. Durusoy, M. Tuncel, A. Tuncel, Monodisperse porous polymer particles with polyionic ligands for ion exchange separation of proteins, Anal. Chim. Acta 570 (2006) 240-248. |
| [12] | F. Gasparrini, D. Misiti, R. Rompietti, C. Villani, New hybrid polymeric liquid chromatography chiral stationary phase prepared by surface-initiated polymerization, J. Chromatogr. A 1064 (2005) 25-38. |
| [13] | S.D. Chambers, C.A. Pohl, C.A. Lucy, Agglomerated carbon based phases for anion exchange chromatography, J. Chromatogr. A 1218 (2011) 263-269. |
| [14] | A.A. Kazarian, M.R. Taylor, P.R. Haddada, P.N. Nesterenkoa, B. Paull, Ion-exchange and hydrophobic interactions affecting selectivity for neutral and charged solutes on three structurally similar agglomerated ion-exchange and mixed-mode stationary phases, Anal. Chim. Acta 803 (2013) 143-153. |
| [15] | Z.P. Huang, L.L. Xi, Q. Subhani, et al., Covalent functionalization of multi-walled carbon nanotubes with quaternary ammonium groups and its application in ion chromatography, Carbon 62 (2013) 127-134. |
| [16] | E.F. Hilder, F. Svec, J.M.J. Fré chet, Latex-functionalized monolithic columns for the separation of carbohydrates by micro anion-exchange chromatography, J. Chromatogr. A 1053 (2004) 101-106. |
| [17] | P. Zakaria, J.P. Hutchinson, N. Avdalovic, Y. Liu, P.R. Haddad, Latex-coated polymeric monolithic ion-exchange stationary phases. 2. Micro-ion chromatography, Anal. Chem. 77 (2005) 417-423. |
| [18] | R.W. Slingsby, C.A. Pohl, Anion-exchange selectivity in latex-based columns for ion chromatography, J. Chromatogr. A 458 (1988) 241-253. |
| [19] | M. Piran, V. Kotlyar, D.D. Medina, et al., End-selective functionalization of carbon nanotubes. Use of DOE for the optimization of a DNA probe attachment and hybridization using an enzymatic amplifying system, J. Mater. Chem. 19 (2009) 631-638. |
| [20] | Q.Y. Yue, Q. Li, B.Y. Gao, Y. Wang, Kinetics of adsorption of disperse dyes by polyepichlorohydrin-dimethylamine cationic polymer/bentonite, Sep. Purif. Technol. 54 (2007) 279-290. |
| [21] | Q. Li, Q.Y. Yue, Y. Su, B.Y. Gao, J. Li, Two-step kinetic study on the adsorption and desorption of reactive dyes at cationic polymer/bentonite, J. Hazard. Mater. 165 (2009) 1170-1178. |
| [22] | Q. Li, Q.Y. Yue, Y. Su, B.Y. Gao, Equilibrium and a two-stage batch adsorber design for reactive or disperse dye removal to minimize adsorbent amount, Bioresour. Technol. 102 (2011) 5290-5296. |
| [23] | Q. Li, Q.Y. Yue, Y. Su, B.Y. Gao, L. Fu, Cationic polyelectrolyte/bentonite prepared by ultrasonic technique and its use as adsorbent for Reactive Blue K-GL dye, J. Hazard. Mater. 147 (2007) 370-380. |
| [24] | Q. Kang, W.Z. Zhou, Q. Li, et al., Adsorption of anionic dyes on poly(epicholorohydrin dimethylamine) modified bentonite in single and mixed dye solutions, Appl. Clay Sci. 45 (2009) 280-287. |
| [25] | D. Gamallo-Lorenzo, M.D.C. Barciela-Alonso, A. Moreda-Piñ eiro, A. Bermejo-Barrera, P. Bermejo-Barrera, Microwave-assisted alkaline digestion combined with microwave-assisted distillation for the determination of iodide and total iodine in edible seaweed by catalytic spectrophotometry, Anal. Chim. Acta 542 (2005) 287-295. |
| [26] | Z.P. Huang, Z.Y. Zhu, Q. Subhani, et al., Simultaneous determination of iodide and iodate in povidone iodine solution by ion chromatography with homemade and exchange capacity controllable columns and column-switching technique, J. Chromatogr. A 1251 (2012) 154-159. |
| [27] | T. Hirokawa, T. Ichihara, K. Ito, A.R. Timerbaev, Trace ion analysis of seawater by capillary electrophoresis: determination of iodide using transient isotachophoretic preconcentration, Electrophoresis 24 (2003) 2328-2334. |
| [28] | W.Z. Hu, P.J. Yang, K. Hasebe, P.R. Haddad, K. Tanaka, Rapid and direct determination of iodide in seawater by electrostatic ion chromatography, J. Chromatogr. A 956 (2002) 103-107. |
| [29] | V.T.P. Nguyen, V. Piersoel, T.E. Mahi, Urine iodide determination by ion-pair reversed-phase high performance liquid chromatography and pulsed amperometric detection, Talanta 99 (2012) 532-537. |
| [30] | A.K. Singh, S. Mehtab, Polymeric membrane sensors based on Cd(II) Schiff base complexes for selective iodide determination in environmental and medicinal samples, Talanta 74 (2008) 806-814. |
| [31] | T.K. Malongo, S. Patris, P. Macours, et al., Highly sensitive determination of iodide by ion chromatography with amperometric detection at a silver-based carbon paste electrode, Talanta 76 (2008) 540-547. |
| [32] | M. Yaqoob, A. Rehman, A. Waseem, A. Nabi, Determination of iodide using flow injection with acidic potassium permanganate chemiluminescence detection, Luminescence 21 (2006) 221-225. |
| [33] | S. Mishra, V. Singh, A. Jain, K.K. Verma, Determination of iodide by derivatization to 4-iodo-N,N-dimethylaniline and gas chromatography-mass spectrometry, Analyst 125 (2000) 459-464. |
| [34] | Q.Y. Yue, B.Y. Gao, Y. Wang, et al., Synthesis of polyamine flocculants and their potential use in treating dye wastewater, J. Hazard. Mater. 152 (2008) 221-227. |