Chiral phenomena are widely known. Many pharmaceutical and bioactive compounds are racemic mixtures with chiral isomers having nearly identical physical and chemical properties. They have different levels of biological activity,toxicity and metabolic mechanisms. The enantiomers have been characterized either directly,or indirectly,using different analytical techniques [1, 2, 3].
The determinating recognition of enantiomers by chiral high performance liquid chromatography (HPLC) has proven to be the most usefulmethod for the analysis of numerous chiral substances [4]. Stereo specificity has been achieved in the ligand-exchange mode by adding chiral reagents directly into the HPLC mobile phase or incorporation into the stationary phase. The a-amino acid may be used as a ligand agent and Cu2+ as a ligand ion [5].
Optical methodologies,especially those that are based on UV and visible spectroscopic techniques,have drawn substantial interests of researchers owing to their advantageous features including simplicity,low cost,high sensitivity,adaptation to automation and real-time analysis,and diverse signal output modes [6]. Optical enantio-sensing has recently emerged as a less labor intensive alternative to chromatographic and optical rotation methods for the assessment of the enantiopurity of a variety of substrate classes [7, 8].
Mandelic acid is an important pharmaceutical intermediate which can be used as a raw material for producing angiotenic agents,including cyclandelate,hydrobenzole and pemoline, bactericide,antispasmodic medications,and so on. Thus,the recognition of the mandelic acid enantiomers is necessary to meet the increasing demands for evaluation of the pharmacokinetic attributes of each enantiomer and to control the enantiomeric purity of pharmaceutical preparations. Several techniques have been proposed for the determination of mandelic acid including nuclearmagnetic resonance spectroscopy [9],fluorescence [10, 11], potentiometry [12],capillary electrophoresis [13] and HPLC [14]. However,these methods are usually time-consuming and quite expensive as a result of the requirements for sophisticated instrumentation. Therefore,simple,inexpensive,and convenient techniques for this purpose are still in demand.
The indicator displacement assay is a typical competitive binding assay constituting a receptor and a signaling unit that also serves as a surrogate substrate [15, 16]. The signaling unit possesses an easily observable and quantifiable property which is modulated in response to competitive binding with an analyte. In this study,the enantioselective association event between the L-Proline-Cu(II) receptor and mandelic acid is exploited to develop an enantioselective indicator displacement assay with the colorimetric indicator pyrocatechol violet (PV). The two coordina- tion sites on Cu(II) are expected to undergo fast and reversible ligand exchange to afford competitive metal coordination for the enantiomeric excess (ee) determination of mandelic acid. This is first report of the enantiodiscrimination of mandelic acid by an indicator displacement assay which combines the convenient technique of spectroscopy and the enantioselectivity offered by chiral ligand-exchange HPLC. The chiral receptor,L-Proline,is readily available and the method possesses many advantages such as a lowprice of the receptor L-Proline,simplicity of operation,high sensitivity of determination,inexpensive apparatus,etc.
2. ExperimentalThe UV/vis spectra were performed using a UV-1600PC spectrophotometer (Mapada instruments,Shanghai,China) equipped with a 10 mm quartz cell. The pH measurements were carried out using a PHS-3C acidity meter.
Pyrocatechol violet (>95%) was purchased from Aladdin (Shanghai,China). The L-Proline (L-Pro) and metal salt Cu(Ac)2 were purchased from Shanghai Chemical Reagent Co.,Ltd. The (+)- and (-)-mandelic acids were obtained from Acros Organics (Geel, Belgium). All other chemicals were of analytical grade. The water used during the experiment was ultrapure water. Mandelic acid was dissolved in methanol-ultrapure water (2:8,v/v) to obtain different concentrations of the stock solution.
Using the PV-L-Pro-Cu(II) ensemble,we detected the colori- metric enantiodiscrimination of mandelic acid in methanol and water (2:8,v/v) solutions. The procedures were as follows: in a pH 6.0 solution containing 0.050 μmol/L PV,0.26 μmol/L Cu(II) and 1.3 μmol/L L-Pro in MeOH:H2O (2:8,v/v); the mandelic acid (pH = 7.0)was gradually titrated into the solution. Simultaneously, changes in the absorption peaks of the solution were monitored using an UV/vis spectrometer.When no further change took place, the titration was terminated and the enantiomeric excess of mandelic acid was thus obtained.
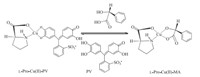
3. Results and discussionIn this study,we sought to achieve enhanced signaling through the use of an indicator displacement assay. The PV indicator effectively competes with the mandelic acid guest for open coordination sites on L-Pro-Cu(II) (Scheme 1). PV is particularly suited to act in this capacity because,upon coordination,it undergoes a large,bathochromic absorbance shift in the visible region,thus providing a highly sensitive and easily observable signal. The chiral recognition mechanism results from the chiral conformation of L-Pro. In order to obtain the stable complex of L-Pro-Cu(II)-PV,we determined the optimized mole ratio of L-Pro:Cu(II):PV was 26:5.2:1. Since the proline is an amphiprotic compound,it may possess some buffering capacity to control the experimental pH value.
|
Download:
|
| Scheme.1.The principle of enantioselective indicator displacement assay. | |
Titration of L-Pro-Cu(II) into PV gave a complex with a shift in lmax resulting in a change in color. Fig. 1(a) shows the change in the UV/vis spectrum when the L-Pro-Cu(II) solution is added into the 0.050 μmol/L PV. With increasing L-Pro-Cu(II) concentration, themaximumabsorption at 445 nmis gradually reduced due to PV decrease and a new absorption peak appears at 615 nm (△μ = 170 nm) owing to L-Pro-Cu(II)-PV generation. The color of the solution changed from yellow to blue in which the PV reacted to form the L-Pro-Cu(II)-PV complex. Fig. 1(b) is the corresponding association isothermat 615 nmfor the addition of L-Pro-Cu(II) (pH 6) into PV (50 μmol/L,pH 6.0) in a 2:8 ratio of MeOH:H2O.

|
Download:
|
| Fig. 1. (a) UV/vis absorption spectra and (b) corresponding association isotherm at 615 nm for the addition of L-Pro-Cu(II) (pH = 6) into PV (50 μmol/L,pH = 6.0) in a 2:8 ratio of MeOH:H2O. | |
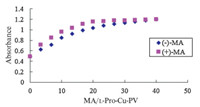
Addition of mandelic acid to the L-Pro-Cu(II)-PV complex resulted in the reverse spectral change,signaling PV displacement. Further,this process occurred with the phenomenon of enantios- electivity observed in Fig. 2,withmore efficient displacement of PV by the (-)-enantiomer. The chiral discrimination was contingent upon the presence of excess L-Pro as in the direct host/guest titration experiments. The enantioselectivity of the response is nearly identical for the mandelic acids with a DA of about 0.12 between the enantiomers. Displacement isotherms for (+)- and (-)-mandelic acid at 615 nm (lmax for L-Pro-Cu(II)-PV complex) are shown in Fig. 2. The experiment proved that the DA value was not obviously affected in the temperature range of 15-25 8C. The minimum detectable concentration for ee of mandelic acid could be calculated from the Fig. 2 to be 0.011 μmol/L.
|
Download:
|
| Fig. 2. Displacement isotherms isothermat 615 nmfor the addition of (+)- and (-)- mandelic acid (pH 7.0) into a pH 6.0 solution containing PV (50 μmol/L) and L-Pro- Cu(II) (260 μmol/L) in a 2:8 ratio of MeOH:H2O. | |
The relationship between ee and absorbance was obtained at constantmandelic acid concentration (5 μmol/L) under conditions that accentuated absorbance differences between the enantio- mers. The DA could be increased to 0.12 by linearly changing the ratio of (+)- and (-)- mandelic acid concentration found to be optimal from the displacement experiments. The resultant ee versus A relationships are remarkably linear. Fig. 3 shows the ee curves for mandelic acid generated with L-Pro-Cu(II)-PV. The repeatability for five replicate determinations was expressed with the error bars. From the calibration curves,the absorbance values of the unknowns may be used to calculate ee values. Therefore, colorimetric enantiodiscrimination of mandelic acid can be obtained.

|
Download:
|
| Fig. 3. Displacement isotherms isothermat 615 nmfor the addition of (+)- and (-)- mandelic acid (pH 7.0) into a pH 6.0 solution containing PV (50 μmol/L) and L-Pro- Cu(II) (260 μmol/L) in a 2:8 ratio of MeOH:H2O. | |
In summary,we have reported an operationally simple sensing scheme based on competitive dynamic metal coordination,which combines the convenient technique of spectroscopy with the enantioselectivity offered by chiral ligand-exchange HPLC. The method allows for the measurement of free mandelic acid enantiomeric excess by visible spectroscopy.
AcknowledgmentThis work was supported by the National Natural Science Foundation of China (No. 21165022).
| [1] | N.M. Maier, P. Franco, W. Lindner, Separation of enantiomers: needs, challenges, perspectives, J. Chromatogr. A 906 (2001) 3-33. |
| [2] | T. Ikai, Y. Okamoto, Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography, Chem. Rev. 109 (2009) 6077-6101. |
| [3] | M. Sohail, Y.F. Wang, S.X. Wu, et al., Non-superimposable mirror image crystals of enantiomers by spontaneous resolution and the chiral discrimination mechanism, Chin. Chem. Lett. 24 (2013) 695-698. |
| [4] | M. Huang, W.J. Chen, Y. Zhou, et al., Enantiomeric separations of four basic drugs containing N-alkyl groups by a RP-HPLC system using SBE-β-CD as chiral mobile phase additive, Chin. Chem. Lett. 24 (2013) 840-844. |
| [5] | A. Kurganov, Chiral chromatographic separations based on ligand exchange, J. Chromatogr. A 906 (2001) 51-71. |
| [6] | J. Su, Y.Q. Sun, F.J. Huo, Y.T. Yang, C.X. Yin, Naked-eye determination of oxalate anion in aqueous solution with copper ion and pyrocatechol violet, Analyst 135 (2010) 2918-2923. |
| [7] | X. Zhang, J. Yin, J. Yoon, Recent advances in development of chiral fluorescent and colorimetric sensors, Chem. Rev. 114 (2014) 4918-4959. |
| [8] | D. Leung, S.O. Kang, E.V. Anslyn, Rapid determination of enantiomeric excess: a focus on optical approaches, Chem. Soc. Rev. 41 (2012) 448-479. |
| [9] | X.X. Liu, Y.S. Zheng, Chiral nitrogen-containing calix[4]crown—an excellent receptor for chiral recognition of mandelic acid, Tetrahedron Lett. 47 (2006) 6357-6360. |
| [10] | F. Miao, J. Zhou, D. Tian, H. Li, Enantioselective recognition of mandelic acid with (R)-1, 1-bi-2-naphthol-linked calix[4]arene via fluorescence and dynamic light scattering, Org. Lett. 14 (2012) 3572-3575. |
| [11] | K. Tanaka, T. Tsuchitani, N. Fukuda, A. Masumoto, R. Arakawa, Highly enantioselective fluorescent recognition of mandelic acid derivatives by chiral salen macrocycles, Tetrahedron Asymm. 23 (2012) 205-208. |
| [12] | L. Xu, Y.Y. Yang, Y.Q. Wang, J.Z. Gao, Chiral salen Mn(III) complex-based enantioselective potentiometric sensor for L-mandelic acid, Anal. Chim. Acta 653 (2009) 217-221. |
| [13] | D.A. Tsioupi, R.I.S. Staden, C.P.K. Christodoulou, Chiral selectors in CE: recent developments and applications, Electrophoresis 34 (2013) 178-204. |
| [14] | Y. Okamoto, E. Yashima, Polysaccharide derivatives for chromatographic separation of enantiomers, Angew. Chem. Int. Ed. 37 (1998) 1020-1043. |
| [15] | J.F. Folmer-Andersen, V.M. Lynch, E.V. Anslyn, Colorimetric enantiodiscrimination of α-amino acids in protic media, J. Am. Chem. Soc. 127 (2005) 7986-7987. |
| [16] | X.F. Mei, C. Wolf, Determination of enantiomeric excess and concentration of unprotected amino acids, amines, amino alcohols, and carboxylic acids by competitive binding assays with a chiral Scandium complex, J. Am. Chem. Soc. 128 (2006) 13326-13327. |




