b Ministry of Education of Fermentation Key Laboratory and Collaboration Innovation Centre of Industrial Fermentation, Hubei University of Technology, Wuhan 430068, China
Cellulase is a complex enzyme consisted of exoglucanase, edoglucanase and β-glucosidase,which synergistically acts to convert cellulose into glucose [1, 2]. By converting cellobiose into glucose,β-glucosidase enhances hydrolysis efficiency [3].
The bottleneck of industrial application is the β-glucosidase cost. A desirable technique to process economy was immobilizing microorganism to produce the enzymes,which has attracted increasing interest. Immobilizing microorganism in gels has been widely employed [4, 5],but the process showed some shortcoming such as toxic effect on the immobilized cells [6, 7],low durability, low mass transfer efficiency and complicate process. Attaching immobilization,which can remarkably reduce the mass transfer resistance [8, 9],is a simpler technique to entangle mycelia.
The chemical carriers of attaching immobilization such as foams,cellulose microfibrils,and so on have been used to immobilize fungi [10, 11],but during their production,environ- ment pollution and cost increasing was unavoidable. A natural carrier took the advantages over the chemical carriers such as being safe to human being,no environment pollution and lower cost. Towel gourd vegetable sponges,which are abundant after the harvest of towel gourd vegetable seeds,are potential for using as a natural carrier.
A valuable application of cellulase or β-glucosidase was hydrolyzing cellulose under high salinity condition,which was detrimental to activity and stability of common enzymes. The b- glucosidase which can show better activity and stability at high salinity was desirable for hydrolyzing cellulose under high salinity condition. The fungi from high salinity condition,seawater,are on the chance of producing the enzymes,which can show better catalysis ability under high salinity condition.
A marine Aspergillus niger has been isolated from the high salinity condition [12]. The investigation proposed here is aimed at efficient producing β-glucosidase by immobilizing the marine A. niger on a novel natural carrier,towel gourd vegetable sponges. Specially,the characteristics of β-glucosidase at high salinity were evaluated too.
2. Experimental 2.1. MicroorganismA marine A. niger was isolated from East China Sea. It was maintained in China Center for Type Culture Collection under the accession number of CCTCCM2010132. The 18S rDNA sequence was submitted to National Center for Biotechnology Information under the accession number of HM446586.
2.2. CarrierMoisture of towel gourd vegetable sponges was 7% (w/w). Diameter of most of towel gourd vegetable sponges wire was between 0.1 cm and 0.2 cm. Density of towel gourd vegetable sponges was 1.65 g/cm3 . Porosity was about 50% (v/v). Towel gourd vegetable sponges were purchased from the local market and cut into 1.5 × 0.5 cm pieces. The pieces were used as a carrier after sterilization at 115 °c for 20 min.
2.3. MediaGermination medium consisted of 150.0 g glucose,3.0 g (NH4)2HPO4,1.0 L artificial seawater,pH 6.5.
Growth medium consisted of 40.0 g glucose,0.3 g KH2PO4 and 0.8 g (NH4)2HPO4 in 1.0 L artificial seawater,2.5 g CaCO3 (sepa- rately sterilized and added to maintain pH between 4.0 and 6.0).
Producing medium consisted of 3.0 g NaNO3,1.0 g KH2PO4, 0.2 g CaCl2,0.5 g peptone,2.0 g Tween 80,0.8 g cellobilose,40.0 g CMC (carboxymethyl cellulose),6.0 g avicel,2.0 g glucose,1.0 L artificial seawater,pH 5.5.
Artificial seawater consisted of 24.54 g/L NaCl,11.10 g/L MgCl2·6H2O,4.09 g/L Na2SO4,1.16 g/L CaCl2,0.69 g/L KCl,0.2 g/L NaHCO3,0.03 g/L H3BO3,0.04 g/L SrCl2·6H2O,0.003 g/L NaF.
2.4. Immobilizing A. nigerAfter incubation on potato dextrose agar (PDA) medium slant for 4 days,the spores maturated. The spores were washed out. The suspension was vigorously stirred and diluted to a final concentration of 4-5 × 107 spores/mL. The spore suspension was repeatedly sprayed on every side of the towel gourd vegetable sponge pieces. After incubation at 37 °c in a culture dish for 32 h, the natural carrier was submerged in growth medium for 30 h. During the carrier was submerged,it was exposed to air for 5 min each 6 h.
2.5. β-glucosidase productionThe cultivation of the immobilized mycelia was conducted in 250 mL flasks and producing medium was filled into the flasks to 30% of their volumes. Ten pieces of carries with immobilized mycelia were put into one flask. The cultivation was at 30 °c and 120 rpm. The repeat batch incubation was carried out in the same condition. Producing medium was changed periodically every 5 days during the repeat batches fermentation by immobilization cultivation.
2.6. Preparation and assay activity of β-glucosidaseFermentation broth was centrifuged at 5000 rpm,and the resultant supernatant was suitably diluted to assay the enzyme activity. One unit of enzyme activity was defined as the amount of enzyme that liberated 2 μmol glucose per minute. β-glucosidase activity was measured using D-salicin as the substrate [13]. DNS method was used to quantify the reducing sugar [14].
2.7. Immobilized biomass percentageDry weight of fungal biomass was determined by filtering culture broth through pre-weighed glass microfilters. The carrier with the attached mycelia was transferred to the flask containing distilled water. The flask was then shaken for 20 min at 250 rpm and 30 °c. The water was collected and the procedure was repeated. The resultant water was filtered through pre-weighed glass microfilters,which was then dried. Dry weight estimation of free biomass wasmeasured. The attachedmycelia were dried with the carrier in a desiccator for 48 h. The measured value for the immobilized biomass was corrected by abstracting the weight of the carrier. The percentage of immobilized biomass was calculated as follows:
where Y% is the ration of immobilized biomass,Mi is the amount of immobilized biomass and Mf is the amount of free biomass. 2.8. Microscopic observationsThe morphology of towel gourd vegetable sponges or A. niger mycelia was observed by a polarized-light microscopy (Nicon).
2.9. Purification of β-glucosidase(NH4)2SO4 was added to the 55% saturation. The precipitation was removed by centrifugation at 10,000 g for 15 min. (NH4)2SO4 was added to the 75% saturation. The precipitation was collected by centrifugation at 10,000 g for 10 min and dissolved in 10.0 mmol/L Tris-HCl buffer. The dissolved samples were concen- trated with ultrafiltrationmembrane (70 kDa cut-off) and dialyzed against the same buffer.
The dialyzed sample was applied to a column of a Q-Sepharose FF Fast Flow (GE Healthcare) equilibrated with 10.0 mmol/L Tris- HCl buffer,pH 7.1. Step elution was undertaken with 10.0 mmol/L Tris-HCl buffer containing 0.2,0.3,0.4,0.5 mol/L NaCl buffer at a flow rate of 3.0 mL/min. The fractions containing β-glucosidase activity from the column were pooled and dialyzed against 10.0 mmol/L Tris-HCl buffer. The dialyzed fractions were applied to a column of Q-Sepharose HP equilibrated with 10.0 mmol/L Tris-HCl buffer,pH 7.1. The column was then eluted with a gradient of 0-0.6 mol/L NaCl at a flow rate of 1.2 mL/min. The active fraction was collected and assayed for β-glucosidase activity.
2.10. Determination half life of β-glucosidase at different salinityThe deactivation of β-glucosidase is assumed to follow first- order kinetics [15]. The equations were expressed as:
where Et is the enzyme activity deactivated for some time. E0 is the initial activity. kd is the first-order deactivation rate constant and t is the time of deactivation of the enzyme. t1/2 is the half life. From the plot of ln(Et/E0) versus t,the slope is the value of Kd. For obtaining the values of (Et/E0) in 0% and 6% NaCl (w/v) solution,the retained activity of purified β-glucosidase was measured after incubation for different time at different temperature. 3. Results and discussion 3.1. Structure of the towel gourd vegetable SpongeThe towel gourd vegetable sponges consisted of filaments, which diameters are between 0.5 mm and 1.0 mm (Fig. 1A). Many holes occurred in the filaments are useful to efficiently immobilize fungi (Fig. 1B). By interlacing each others,the filaments of towel gourd vegetable sponges exhibited morphology similar to matrix- es. Crystallinity and compaction of towel gourd vegetable sponges is high,so it can maintain good stability during five batches. It illustrated that the natural carrier was suitable to be used as a carrier.

|
Download:
|
| Fig.1. Themorphology of towel gourd vegetable sponges and immobilizedmycelia for different cultivation time. (A) Interlacing filaments of towel gourd vegetable sponges. (B) The morphology of single filaments of towel gourd vegetable sponges. (C) The morphology of the mycelia immobilized on towel gourd vegetable sponges cultivated for 32 h in a culture dish. (a) The fungal mycelia; (b) the filament of towel gourd vegetable sponges. (D) The morphology of the lay of the fungal mycelia immobilized on towel gourd vegetable sponge after the second repeat batch cultivation. (c) The layer of fungal mycelia; (d) the filament of towel gourd vegetable sponges. (E) The enlarged morphology of the fungal mycelia immobilized on towel gourd vegetable sponges cultivated after first repeat batch cultivation. (F) The morphology of the lay of the fungal mycelia immobilized on towel gourd vegetable sponges cultivated for sixth repeat batch cultivation. (e) The filament of towel gourd vegetable sponges covered by the fungal mycelia; (f) the layer of fungal mycelia. | |
Incubating for 32 h in culture dishes, the mycelia attached tightly on the filaments of towel gourd vegetable sponges (Fig. 1C). The mycelia could easily cover the whole surface of the carriers.
3.3. Morphology of the mycelia during repeat batch fermentationAfter the second batch, the mycelia layers become thicker and covered the total surface of the carrier (Fig. 1D). For observing the morphology of mycelia attaching on the carrier, the mycelia layer was partially enlarged to observe the details. The observation showed that the mycelia were still strong (Fig. 1E and F). Themarine A. niger could be effectively immobilized on the natural carrier of towel gourd vegetable sponges.
3.4. The immobilizing ability of natural towel gourd vegetable spongesFor analyzing the ability of immobilizing biomass, the immobilized A. niger was cultivated in germination medium with sufficient nutrition, which contained 150 g/L glucose. Biomass amount increased rapidly and the amount of the immobilized biomass achieves 0.148 g/g towel gourd vegetable sponges or 2.96 g/L (Fig. 2). The immobilized biomass percentage was over 90% after cultivation for 48 h. The ability of immobilizing biomass was comparable to that of other kinds of chemical carriers [11]. The natural carrier showed the good ability of immobilizing sufficient biomass to possess high productivity.

|
Download:
|
| Fig.2. The time course of the immobilized biomass and immobilized biomass percentage. | |
Although the novel natural carrier showed good ability of immobilizing biomass under sufficient rich nutrition condition, the ability of immobilizing biomass in producing medium, which nutrition was not so sufficient, should be tested. The biomass increased and the immobilized biomass percentage was over 95% (Fig. 3). Natural towel gourd vegetable spongeswere a good carrier to immobilize A. niger under producing condition.

|
Download:
|
| Fig.3. The time course of the immobilized biomass and percentage during the six repeat batch cultivation. | |
Fermentation of consecutive six batches with the mycelia immobilized on the natural carries could successfully be con- ducted, which was comparable to that with the mycelia immobilized in gel [7]. Although the production by the immobi- lized mycelia were less than that by free mycelia at the first or second and fifth batches, the repeat production by the immobilized mycelia were still desirable, over 110 U/mL. The third or fourth production by the immobilized fungus was higher than that by the free mycelia (Fig. 4). The production by the free or immobilized mycelia was higher than the reported [16–18]. The maximum production of β-glucosidase occurred 1.5 days earlier by the immobilized mycelia than by the free. The immobilization process enhanced the production and productivity.

|
Download:
|
| Fig.4. The time course of b-glucosidase production of the six repeat batches cultivation. | |
The activity of β-glucosidase was analyzed in 0%, 3%, 6% and 9% NaCl (w/v) solution. The activity in NaCl free solution was the minimum. The activity in 6% NaCl (w/v) solution is about 1.46 fold higher than that in NaCl free solution.
The longer the half-life of the enzyme is, the more stable the enzyme is [6]. The half-life in 6% NaCl solution was twice longer than that in NaCl free solution. It illustrated that the β-glucosidase from the marine fungus was more stable at high salinity (Fig. 5). The β-glucosidase, which showed better stability and higher activity at high salinity was valuable for hydrolyze cellulose under high salinity condition such as black liquor, waste water from acidic or alkaline treatment process to cellulosicmaterial, cellulose in marine algae, marine litters, and so on [19–21].

|
Download:
|
| Fig.5.The halflifes and activity of the β-glucosidase at different salinity. | |
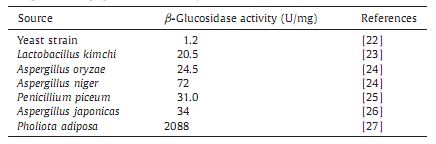
Catalysis ability of different kinds of β-glucosidase showed great difference, showed in Table 1. β-glucosidase from A. niger was better than that from yeast, Lactobacillus kimchi. b-Glucosi- dase from Pholiota adiposa showed the highest activity. The primary advantage of β-glucosidase from the marine A. niger was to hydrolyze cellulose under high salinity.
| Table 1 Comparision of β-glucosidase activity from different strains. |
By immobilizing a marine A. niger on a novel natural carrier, towel gourd vegetable sponges, β-glucosidase productionwas over 100 U/mL during five batches. The maximum production by immobilized mycelia occurred 1.5 day earlier than by the free mycelia. Interestingly, β-glucosidase exhibited better catalysis ability under high salinity condition.
| [1] | U. Hö lker, M. Hö lker, J. Lenz, Biotechnological advantages of laboratory-scale solid-state fermentation with fungi, Appl. Microbiol. Biotechnol. 64 (2004) 175-186. |
| [2] | J.P.H. Van Wyk, Biotechnology and the utilization of biowaste as a resource for bioproduct development, Trends Biotechnol. 19 (2001) 172-177. |
| [3] | G. Rastogi, A. Bhalla, A. Adhikari, et al., Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains, Bioresour. Technol. 101 (2010) 8798-8806. |
| [4] | M. Quirasco, M. Remaud-Simeon, P. Monsan, A. Ló pez-Munguía, Experimental behavior of a whole cell immobilized dextransucrase biocatalyst in batch and packed bed reactors, Bioprocess Biosyst. Eng. 20 (1999) 289-295. |
| [5] | I. Virkajä rvi, M. Linko, Immobilization: a revolution in traditional brewing, Naturwissenschaften 86 (1999) 112-122. |
| [6] | C. Roisin, C. Bienaime, J.E. Nava Saucedo, J.-N. Barbotin, Influence of the microenvironment in immobilized Gibberella fujikuroi, in: R.H. Wijffels, R.M. Buitelaar, C. Bucke, J. Tramper (Eds.), Immobilized Cells Basics and Applications, Elsevier, Amsterdam, 1996, pp. 189-195. |
| [7] | K.A. Lusta, N.G. Starostina, B.A. Fikhte, Immobilization of microorganisms: cytophysiological aspects, in: J.A.M. de Bont, J. Visser, B. Mattiasson, J. Tramper (Eds.), Proceedings of an International Symposium: Physiology of Immobilized Cells, Elsevier, Amsterdam, 1990, pp. 557-562. |
| [8] | T.W. Chiou, Y.C. Wang, H.S. Liu, Utilizing the macroporous packed bed for insect cell/baculovirus expression. Part 2: the production of human interleukin-5 in polyurethane foam and cellulose foam packed bed bioreactors, Bioprocess Eng. 18 (1998) 91-100. |
| [9] | C. Lapadatescu, G. Feron, C. Vergoignan, et al., Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens, Appl. Microbiol. Biotechnol. 47 (1997) 708-714. |
| [10] | N.V. Sankpal, A.P. Joshi, B.D. Kulkarni, Citric acid production by Aspergillus niger immobilized on cellulose microfifibrils: influence of morphology and fermenter conditions on productivity, Process Biochem. 36 (2001) 1129-1139. |
| [11] | N.V. Sankpal, B.D. Kulkarni, Optimization of fermentation conditions for gluconic acid production using Aspergillus niger immobilized on cellulose microfibrils, Process Biochem. 37 (2002) 1343-1350. |
| [12] | D.S. Xue, H.Y. Chen, D.Q. Lin, Y.X. Guan, S.J. Yao, Optimization of a natural medium for cellulase by a marine Aspergillus niger using response surface methodology, Appl. Biochem. Biotechnol. 167 (2012) 1963-1972. |
| [13] | D.S. Chahal, Solid-state fermentation with Trichoderma reesei for cellulase production, Appl. Environ. Microbiol. 49 (1985) 205-210. |
| [14] | G.L. Miller, Use of dinitrosalicylic acid reagent for determination of reducing sugar, Anal. Chem. 31 (1959) 426-428. |
| [15] | K.S. Siddiqui, A.A.N. Saqib, M.H. Rashid, M.I. Rajoka, Thermostabilization of carboxymethylcellulase from Aspergillus niger by carboxyl group modification, Biotechnol. Lett. 19 (1997) 325-330. |
| [16] | A.-R. Joo, M. Jeya, K.-M. Lee, et al., Production and characterization of β-1,4-glucosidase from a strain of Penicillium pinophilum, Process Biochem. 45 (2010) 851-858. |
| [17] | S.W. Kang, Y.S. Park, J.S. Lee, S.I. Hong, S.W. Kim, Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass, Bioresour. Technol. 91 (2004) 153-156. |
| [18] | Z.Y. Wen, W. Liao, S.L. Chen, Production of cellulase/β-glucosidase by the mixed fungi culture Trichoderma reesei and Aspergillus phoenicis on dairy manure, Process Biochem. 40 (2005) 3087-3094. |
| [19] | P. Beguin, J.-P. Aubert, The biological degradation of cellulose, FEMS Microbiol. Rev. 13 (1994) 25-58. |
| [20] | J.M. Lawther, R.C. Sun, W.B. Banks, Extraction, fractionation and characterization of structural polysaccharides from wheat straw, J. Agric. Food Chem. 43 (1995) 667-675. |
| [21] | X.D. Liu, X. Wang, Study on pretreatment of crop straw, Food Fermentat. Ind. 34 (2008) 110-114. |
| [22] | Z.L. Liu, S.A. Weber, M.A. Cotta, S.Z. Li, A new β-glucosidase producing yeast for lower-cost cellulosic ethanol production from xylose-extracted corncob residues by simultaneous saccharification and fermentation, Bioresour. Technol. 104 (2012) 410-416. |
| [23] | J.-A. Ko, J.Y. Park, H.J. Kwon, et al., Purification and functional characterization of the first stilbene glucoside-specific β-glucosidase isolated from Lactobacillus kimchi, Enzyme Microb. Technol. 67 (2014) 59-66. |
| [24] | C.Z. Zhang,D. Li, H.S.Yu,B.Zhang, F.X. Jin,Purificationand characterizationof piceidβ-D-glucosidase from Aspergillus oryzae, Process Biochem. 42 (2007) 83-88. |
| [25] | L. Gao, F. Gao, X.K. Jiang, et al., Biochemical characterization of a new β-glucosidase (Cel3E) from Penicillium piceum and its application in boosting lignocelluloses bioconversion and forming disaccharide inducers: new insights into the role of β-glucosidase, Process Biochem. 49 (2014) 768-774. |
| [26] | T.M. Silva, B.C. Pessel, C.R. Jean Silva, et al., Immobilization and high stability of an extracellular β-glucosidase from Aspergillus japonicus by ionic interactions, J. Mol. Catal. B: Enzym. 104 (2014) 95-100. |
| [27] | S.S. Jagtap, S.S. Dhiman, T.-S. Kim, et al., Characterization of a β-1,4-glucosidase from a newly isolated strain of Pholiota adiposa and its application to the hydrolysis of biomass, Biomass Bioener. 54 (2013) 181-190. |