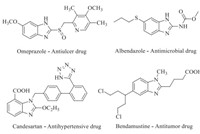
At the beginning of this century,green chemistry has attracted a considerable importance in the development of environmentally benign routes to numerous materials. Green chemistry mainly emphasizes towards the pollution prevention through eco-friendly design of chemical products and processes [1]. The development of greenermethodologies for syntheses of heterocyclic compounds is still a stimulating task in the field of organic synthesis. Among the heterocycles,benzimidazole derivatives are the important class of nitrogen containing heterocycles with a wide range of medicinal properties such as serotoninergic 5-HT3 and 5-HT4 receptors in the CNS [2],antihistamine [3],anticancer [4, 5],antibacterial [6], antifungal [7],anti-inflammatory,antianalgesic [8],antioxidant [9],antidiabetic [10],selective neuropeptide YY1 receptor antagonists [11],antimalerial,antitubercular [12],antiulcer [13], etc.wheremoiety plays the role of ‘Master Key’ [14]. Therefore,it is an imperative anchor for development of newtherapeutic drugs,as illustrated and supported by some commercial benzimidazole products in Fig. 1.
|
Download:
|
| Fig. 1. Benzimidazole containing important commercial drugs. | |
Generally,the synthesis of benzimidazole involves the reaction of o-phenylenediamine either with carboxylic acids,carboxalde- hydes or their derivatives (chlorides,nitriles,and orthoesters)under strongly acidic conditions with high temperature [15], Furthermore,Cascade reactions of o-haloaniline with amidine hydrochlorides [16] and intramolecular palladium-catalyzed aryl amination are alternative ways for synthesis of benzimidazole [17, 18]. A variety of catalysts are reported in the benzimidazole synthesis,such as FeCl3-doped polyaniline nanoparticles [19], solvent free SiO2/ZnCl2 [20],cobalt (II) chloride hexahydrate [21], [Sm(OTf)3] [22],[In(OTf)3] [23],sodium metabisulfite [24], silphox[POCl3-n (SiO2)n] [25],potassium persulfate-CuSO4 [26], indion 190 resin [27],ammonium acetate [28],thiamine hydro- chloride [29],SDS micelles,DBSA,Fe3O4@SiO2@(NH4)6-Mo7O24 magnetic core-shell nanocomposite,boron trifluoride etherate (BF3.OEt2),Cu-nanoparticles/SiO2,LiBr [30],etc.
At present,bismuth (III) compounds have recently attracted much attention in organic transformations due to their high acidity,thermal stability,low toxicity,low cost,and good stability [31]. Furthermore,bismuth nitrate is reported as an eco-friendly nitrating agent for selective nitration of organic compounds [32, 33]. Current literature reveals that bismuth nitrate has been utilized as an effective catalyst in the synthesis of 3,4-dihydropyr- imidin-2(1H)-ones [34],guanidylation of N-benzoylthioureas [35], synthesis of coumarins [36],Paal-Knorr synthesis of pyrroles [37], chemoselective synthesis of acylals [38],etc.
Nevertheless,most of the aforesaid methods of benzimidazole synthesis have disadvantages like,use of expensive reagents and catalysts,harsh reaction conditions and long reaction time,etc. Moreover,several of these reactions have been reported at higher temperatures which are not accepted as environmentally friendly. Therefore,the search continues for a better catalyst to synthesize benzimidazoles in term of operational simplicity. To address this problem,in our present research investigation,we wish to report bismuth nitrate as an efficient catalyst for synthesis of 2- substituted benzimidazoles and 1,2-disubstituted benzimidazoles. Very interestingly,herein,we revealed that a change of substitu- ent,specifically a replacement of C3 or C4 hydrogen by either hydroxyl or methoxy group on the aldehyde unit,dramatically influences the course of the reaction.
2. Experimental 2.1. Chemicals and instrumentsAll chemicals and materials are procured from S. D. Fine Chemicals Ltd. and Spectrochem Chemicals Pvt. Ltd. and used without further purification.Melting points were determined with open capillary method and are uncorrected. IR spectra were recorded in KBr on Shimadzu IR Affinity-1 FT-IR spectrophotome- ter and 1 H NMR spectra were recorded on a Bruker Avance II 300 and 400 MHz NMR spectrophotometer in CDCl3/DMSO using TMS as internal standard.Mass spectra were recorded onWaters,Q-Tof Micromass (LCMS) spectrometer and Varian Inc. 410 Prostar Binary LC with 500 Mass Spectrophotometer.
2.2. General procedure for the synthesis of 2-substituted benzimidazoles and 1,2- disubstituted benzimidazolesA mixture of o-phenylenediamine (1 mmol),ethanol (5 mL), and bismuth nitrate (10 mol%) was taken in a round-bottom flask. To this mixture,a solution of aldehyde 1.1 mmol for the synthesis of 2-substituted benzimidazoles and 2.1 mmol for the synthesis of 1,2 disubstituted benzimidazole in ethanol (5 mL) was added dropwise with stirring and stirring was continued until the completion of reaction at room temperature. After completion of the reaction (monitored by TLC,hexane: ethyl acetate),the reaction mixture was poured into crushed ice to give solid product,which was filtered,washed with water and dried. The crude product was recrystallized from ethanol to afford pure 2-substituted benzimidazoles or 1,2-disubstituted benzimida- zoles in good to better yields. Spectroscopic data for all the synthesized compounds are depicted in supplementary data, which is in harmony with the structures.
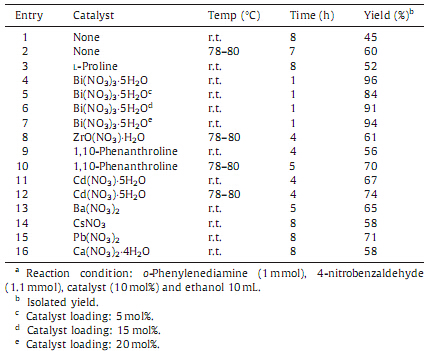
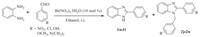
3. Results and discussionIn this study,we examined the synthesis of 2-substituted benzimidazoles by the reaction of o-phenylenediamine with 4- nitrobenzaldehyde using bismuth nitrate as catalyst in ethanol at room temperature. The reaction was completed within 60 min to give the 2-(4-nitrophenyl)-1H-benzimidazole as a product with quantitative yield (Scheme 1). Encouraged by this result,we studied different parameters of reaction and the obtained results are summarized in Tables 1-3. In order to find out optimum reaction condition for the synthesis of 2-substituted benzimid- azole,a separate study with different catalysts and solvents was performed. Bismuth nitratewas found to be an efficient catalyst for synthesis of 2-substituted benzimidazoles over the other catalyst studied.
|
Download:
|
| Scheme.1. Bismuth nitrate mediated synthesis of 2-substituted benzimidazole derivatives. | |
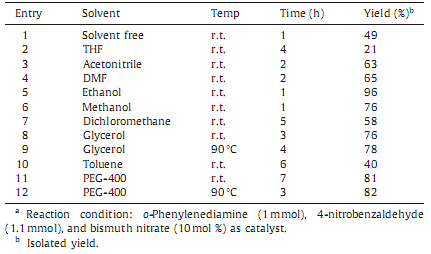
In order to study the performance of catalyst,a controlled reaction was performed using o-phenylenediamine with 4- nitrobenzaldehyde in ethanol without catalyst. The percentages of products formed under controlled condition and with the different catalysts are summarized in Table 1. The results revealed that,under the controlled condition the percentage of products were less even after 8 h in most of the cases,whereas in the presence of bismuth nitrate the obtained yield was highest within a short reaction period of 1 h only. Amidst,in order to get more insides about the amount of catalyst required for the efficient conversion,we performed the experiments by using different percentage loading of catalyst in ethanol at room temperature (Table 1,entries 4-7). The experimentation revealed that 10 mol % loading of catalyst afforded the desired productswith highest yield just within 1 h. Thus,it was found that bismuth nitrate plays a benign role of accelerator promoting the time and cost effective formation of product. In order to study the effect of solvents over the oxidative coupling of 4-nitrobenzaldehyde with o-phenylene- diamine,we carried out the reaction in different solvents. Less polar and aprotic solvents like dichloromethane,toluene and tetrahydrofuran were found to be unsuitable for the reactions,whereas,more polar and protic solvents like ethanol,glycerol, polyethylene glycol (PEG),etc.,were appropriate solvents to afford the higher yields (Table 2).
| Table 1 Effect of catalyst on synthesis of 2-(4-nitrophenyl) benzimidazole.a. |
| Table 2 Effect of solvent on synthesis of 2-(4-nitrophenyl)benzimidazole.a. |
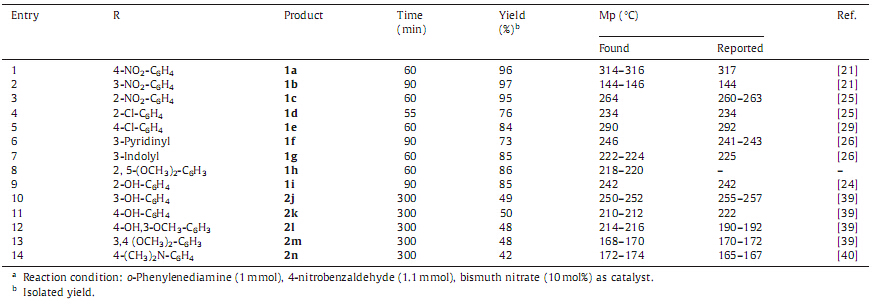
Under the optimized reaction conditions,we performed the reactions of aromatic and heteroaromatic aldehydes with o- phenylenediamine to give quantitative yields of products (Table 3). In some reactions,dialdimineswere formed as byproducts (1%-5%) which were separated during recrystallization. Several functional groups such as Cl,Br,NO2,OCH3 and sensitive heterocyclic molecules like indole-3-carboxaldehyde and pyridyl-3-carboxal- dehyde were compatible with the reaction conditions. All the synthesized compounds were isolated,purified and characterized by FT-IR, 1 H NMR,and LC-MS spectroscopic techniques. In 1 H NMR analysis (Supporting information),the compound (2n) shows the two sharp singlets for -NCH3. This is due to conjugation of unpaired electrons on the nitrogen atomwith aromatic π electrons and this conjugation makes a partial double bond between ipso carbon and nitrogen atom.
| Table 3 Synthesis of benzimidazole derivatives from o-phenylenediamine and aldehydesa. |
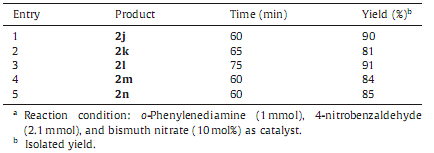
From the above data,it was observed that all the reactions would have offered the expected corresponding 2-substituted benzimidazoles,but surprisingly the reactions with aromatic aldehydes carrying either hydroxyl or methoxy groups at meta or para positions,resulted in the formation of 1,2-disubstituted benzimidazoles. As per the optimized conditions,we have used o-phenylenediamine: aldehydes (1:1.1 mmol),and found that,still after 5 h o-phenylenediamine was unreacted whereas other precursor,i.e. aldehyde gets completely consumed. After isolation and purification of product,it was observed that the obtained product was 1,2-disubstituted benzimidazoles and not expected 2-substituted benzimidazole.With this observation,we planned to set the reactions with two equivalents of aldehydes and one equivalent of o-phenylenediamine under the optimized condi- tions,where 1,2-disubstituted benzimidazoles were obtained as major product with excellent yields (Table 4,Scheme 2).
| Table 4 Synthesis of 1,2-disubstituted benzimidazole derivatives from o-phenylenedia-mine and aldehydesa.. |

|
Download:
|
| Scheme.2. Bismuth nitrate mediated synthesis of 1,2-disubstituted benzimidazole derivatives. | |
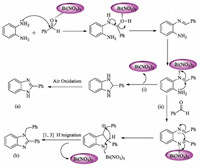
3.2. Plausible mechanism
At the initial stage of synthesis of 2-substituted benzimidazoles, bismuth nitrate forms hydrogen bonding with aldehyde which leads to the activation of carbonyl carbon and subsequently facile attack of diamine over aldehyde forms 2-substituted benzimida- zoles. Furthermore,In the synthesis of 1,2-disubstituted benzi- midazoles,aldehyde initially reacted with diamine to form Schiff base as an intermediate in the presence of electrophilic catalyst, followed by intramolecular 1,3-hydride migration led to the formation of 1,2-disubstituted benzimidazoles (Scheme 3).
|
Download:
|
| Scheme.3. Plausible mechanism for synthesis of (i) 2-substituted benzimidazoles (a) and (ii) 1,2-disubstituted benzimidazoles (b). | |
In summary,bismuth nitrate played a crucial role of mild and efficient green catalyst for the syntheses of 2-substituted and 1,2- disubstituted benzimidazoles in quantitative yields from o-pheny- lenediamine and a wide variety of aldehydes. This methodology followed column chromatography-free protocol availing the abol- ishment of large amount of organic solvents. A one-pot synthesis with short reaction time; use of inexpensive,non-toxic,and easily available catalyst; easy isolation of products with higher yields are the key leads of the methodology executed herein. Thus,this work furthered a green,time aswell as cost effective route to researcher’s further dealing with these scaffolds in future.
AcknowledgmentsAuthors are thankful to UGC,New Delhi,India for financial assistance under UGC-Major Research Project [No. 41-302/2012 (SR)] and SAIF,Panjab University,Chandigarh for NMR analysis.
Appendix A. Supplementary dataSupplementarymaterial related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.012.
| [1] | P.T. Anastas, J.C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998. |
| [2] | L. Maria, L. Rodriguez, B.M. Bellinda, et al., Benzimidazole derivatives. 2. Synthesis and structure-activity relationships of new azabicyclic benzimidazole-4-carboxylic acid derivatives with affinity for serotoninergic 5-HT3 receptors, J. Med. Chem. 42 (1999) 5020-5028. |
| [3] | (a) M.L. Richards, S.C. Lio, A. Sinha, K.K. Tieu, J.C. Sircar, Novel 2-(substituted phenyl)benzimidazole derivatives with potent activity against IgE, cytokines, and CD23 for the treatment of allergy and asthma, J. Med. Chem. 47 (2004) 6451-6454; (b) X.J. Wang, M.Y. Xi, J.H. Fu, et al., Synthesis, biological evaluation and SAR studies of benzimidazole derivatives as H1-antihistamine agents, Chin. Chem. Lett. 23 (2012) 707-710. |
| [4] | W.A. Craigo, B.W. Lesueur, E.B. Skibo, Design of highly active analogues of the pyrrolo[1,2-a]benzimidazole antitumor agents, J. Med. Chem. 42 (1999) 3324-3333. |
| [5] | (a) Y. Tong, J.J. Bouska, P.A. Ellis, et al., Synthesis and evaluation of a new generation of orally efficacious benzimidazole-based poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors as anticancer agents, J. Med. Chem. 52 (2009) 6803-6813; (b) A. Sharma, V. Luxami, K. Paul, Synthesis, single crystal and antitumor activities of benzimidazole-quinazoline hybrids, Bioorg. Med. Chem. Lett. 23 (2013) 3288-3294; (c) X.J. Wang, M.L. Yang, L.P. Zhang, et al., Design of novel bis-benzimidazole derivatives as DNA minor groove binding agents, Chin. Chem. Lett. 25 (2014) 589-592. |
| [6] | (a) C.N. Raut, S.M. Bagul, R.A. Janrao, et al., Synthesis of some novel N-alkyl/ acyl/aroyl 2-(chroman/6-bromochroman-2-yl)-1H-benzimidazoles using ionic liquids and their antibacterial activity, J. Heterocycl. Chem. 47 (2010) 582-588; (b) C.N. Raut, S.M. Bharambe, Y.A. Pawar, P.P. Mahulikar, Microwave-mediated synthesis and antibacterial activity of some novel 2-(substituted biphenyl) benzimidazoles via Suzuki-Miyaura cross coupling reaction and their Nsubstituted derivatives, J. Heterocycl. Chem. 48 (2011) 419-425; (c) S. Ozden, D. Atabey, S. Yildiz, H. Goker, Synthesis and potent antimicrobial activity of some novel methyl or ethyl 1H benzimidazole-5 carboxylates derivatives carrying amide or amidine groups, Bioorg. Med. Chem. 13 (2005) 1587-1597; (d) P.Z. Zhang, S.F. Zhou, T.R. Li, L. Jiang, Efficient synthesis and in vitro antifungal activity of 1H-benzimidazol-1-yl acetates/propionates containing 1H-1,2,4-triazole moiety, Chin. Chem. Lett. 23 (2012) 1381-1384; (e) H.H. Jardosh, B.S. Chetan, M.P. Patel, R.G. Patel, One step synthesis of pyrido[1,2 a]benzimidazole derivatives of aryloxypyrazole and their antimicrobial evaluation, Chin. Chem. Lett. 24 (2013) 123-126; (f) U. Yilmaza, H. Kucukbaya, N. Sireci, et al., Synthesis, microwave-promoted catalytic activity in Suzuki-Miyaura cross-coupling reactions and antimicrobial properties of novel benzimidazole salts bearing trimethylsilyl group, Appl. Organometal. Chem. 25 (2011) 366-373. |
| [7] | H. Kucukbaya, R. Durmaz, N. Okyucu, S. Gunald, Antifungal activity of some bis-5-methylbenzimidazole compounds, Folia Microbiol. 48 (5) (2003) 679-681. |
| [8] | (a) S.M. Sondhi, N. Singh, A. Kumar, O. Lozachc, L. Meijer, Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff's bases, Bioorg. Med. Chem. 14 (2006) 3758-3765; (b) R.K. Arora, N. Kaur, Y. Bansal, G. Bansal, Novel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agents, Acta Pharm. Sin. B 4 (5) (2014) 368-375. |
| [9] | C. Kus, G.A. Kilcigil, S. Ozbey, et al., Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class, Bioorg. Med. Chem. 16 (2008) 4294-4303. |
| [10] | R. Vinodkumar, S.D. Vaidya, B.V.S. Kumar, et al., Synthesis, anti-bacterial, antiasthmatic and anti-diabetic activities of novel N-substituted-2-(4-phenylethynyl-phenyl)-1H-benzimidazoles and N-substituted 2[4-(4,4-dimethyl-thiochroman-6-yl-ethynyl)-phenyl)-1H benzimidazoles, Eur. J. Med. Chem. 43 (2008) 986-995. |
| [11] | J. Camacho, A. Barazarte, A. Gamboa, et al., Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial, cytotoxic and antitubercular agents, Bioorg. Med. Chem. 19 (2011) 2023-2029. |
| [12] | K.S. Jain, A.K. Shah, J. Bariwal, et al., Recent advances in proton pump inhibitors and management of acid-peptic disorders, Bioorg. Med. Chem. 15 (2007) 1181-1205. |
| [13] | Y. Bansal, O. Silakari, The therapeutic journey of benzimidazoles: a review, Bioorg. Med. Chem. 20 (2012) 6208-6236. |
| [14] | (a) L.M. Dudd, E. Venardou, E.G. Verdugo, et al., Synthesis of benzimidazoles in high-temperature water, Green Chem. 5 (2003) 187-192; (b) P.N. Preston, Benzimidazoles and congeneric tricyclic compounds, in: A. Weissberger, E.C. Taylor (Eds.), The Chemistry of Heterocyclic Compounds, Part 1, vol. 40, Wiley, New York, 1981, pp. 6-60; (c) M.R. Grimmett, Imidazoles and their benzo derivatives, in: A.R. Katritzky, C.W. Rees (Eds.), Comprehensive Heterocyclic Chemistry, Pergamon, Oxford, 1984, pp. 457-487;(d) S.K. Rangappa, K.M. Hosamani, H.R.S. Reddy, R.V. Shingalapur, Wells-Dawson heteropolyacid: an efficient recyclable catalyst for the synthesis of benzimidazoles under microwave condition, Catal. Lett. 131 (2009) 552-559;(e) H. Thakuria, G. Das, An expeditious one-pot solvent-free synthesis of benzimidazole derivatives, ARKIVOC 15 (2008) 321-328. |
| [15] | Y. Qu, L. Pan, Z. Wu, X. Zhou, Synthesis of benzimidazoles by Cu2O catalyzed cascade reactions between o-haloaniline and amidine hydrochlorides, Tetrahedron 69 (2013) 1717-1719. |
| [16] | C.T. Brain, S.A. Brunton, An intramolecular palladium-catalysed aryl amination reaction to produce benzimidazoles, Tetrahedron Lett. 43 (2002) 1893-1895. |
| [17] | G. Brasche, S.L. Buchwald, C-H Functionalization/C-Nbond formation: copper-catalyzed synthesis of benzimidazoles from amidines, Angew. Chem. 120 (2008) 1958-1960. |
| [18] | M.A. Alibeik, M. Moosavifard, FeCl3-doped polyaniline nanoparticles as reusable heterogeneous catalyst for the synthesis of 2-substituted benzimidazoles, Synth. Commun. 40 (2010) 2686-2695. |
| [19] | R.G. Jacob, L.G. Dutra, C.S. Radatz, et al., Synthesis of 1,2-disubstitued benzimidazoles using SiO2/ZnCl2, Tetrahedron Lett. 50 (2009) 1495-1497. |
| [20] | A.T. Khan, T. Parvin, L.H. Choudhury, A simple and convenient one-pot synthesis of benzimidazole derivatives using cobalt(II)chloride hexahydrate as catalyst, Synth. Commun. 39 (2009) 2339-2346. |
| [21] | V. Narsaiah, A.R. Reddy, J.S. Yadav, Mild and highly efficient protocol for the synthesis of benzimidazoles using samarium triflate [Sm(OTf)3], Synth. Commun. 41 (2011) 262-267. |
| [22] | R. Trivedi, S.K. De, R.A. Gibbs, A convenient one-pot synthesis of 2-substituted benzimidazoles, J. Mol. Catal. A: Chem. 245 (2006) 8-11. |
| [23] | G.N. Vazquez, H.M. Diaz, S.E. Soto, et al., Microwave-assisted one-pot synthesis of 2-(substituted phenyl)-1H-benzimidazole derivatives, Synth. Commun. 37 (2007) 2815-2825. |
| [24] | A. Hasaninejad, K. Niknam, A. Zare, E. Farsimadan, M. Shekouhy, Silphox [POCl3-n (SiO2)n] as a new, efficient, and heterogeneous reagent for the synthesis of benzimidazole derivatives under microwave irradiation, Phosphorus Sulfur Silicon Relat. Elem. 184 (2009) 147-155. |
| [25] | A. Kumar, R.A. Maurya, D. Saxena, Diversity-oriented synthesis of benzimidazole, benzoxazole, benzothiazole and quinazolin-4(3H)-one libraries via potassium persulfate-CuSO4-mediated oxidative coupling reactions of aldehydes in aqueous micelles, Mol. Divers. 14 (2010) 331-341. |
| [26] | V.S. Padalkar, V.D. Gupta, K.R. Phatangare, et al., Indion 190 resin: efficient, environmentally friendly, and reusable catalyst for synthesis of benzimidazoles, benzoxazoles, and benzothiazoles, Green Chem. Lett. Rev. 5 (2012) 139-145. |
| [27] | H. Sharghi, O. Asemani, R. Khalifeh, New one-pot procedure for the synthesis of 2-substituted benzimidazoles, Synth. Commun. 38 (2008) 1128-1136. |
| [28] | M. Lei, L. Ma, L. Hu, One-pot synthesis of 1H-benzimidazole derivatives using thiamine hydrochloride as a reusable organocatalyst, Synth. Commun. 42 (2012) 2981-2993. |
| [29] | (a) K. Bahrami, M.M. Khodaei, A. Nejati, Synthesis of 1,2-disubstituted benzimidazoles, 2-substituted benzimidazoles and 2-substituted benzothiazoles in SDS micelles, Green Chem. 12 (2010) 1237-1241; (b) V. Kumar, D.G. Khandare, A. Chatterjee, M. Banerjee, DBSA mediated chemoselective synthesis of 2-substituted benzimidazoles in aqueous media, Tetrahedron Lett. 54 (2013) 5505-5509; (c) G. Bai, X. Lan, X. Liu, et al., An ammonium molybdate deposited amorphous silica coated iron oxide magnetic core-shell nanocomposite for the efficient synthesis of 2-benzimidazoles using hydrogen peroxide, Green Chem. 16 (2014) 3160-3168; (d) Y.K. Bommegowda, G.S. Lingaraju, S. Thamas, et al., Weinreb amide as an efficient reagent in the one pot synthesis of benzimidazoles and benzothiazoles, Tetrahedron Lett. 54 (2013) 2693-2695; (e) S.M. Inamdar, V.K. More, S.K. Mandal, CuO nano-particles supported on silica, a new catalyst for facile synthesis of benzimidazoles, benzothiazoles and benzoxazoles, Tetrahedron Lett. 54 (2013) 579-583; (f) D.V. Dekhane, S.S. Pawar, S.V. Gupta, M.S. Shingare, S.N. Thore, Lithium bromide catalyzed solvent free method for synthesis of 2-substituted benzimidazoles and imidazopyridines, Chin. Chem. Lett. 21 (2010) 519-523. |
| [30] | J.M. Bothwell, S.W. Krabbez, R.S. Mohan, Applications of bismuth (III) compounds in organic synthesis, Chem. Soc. Rev. 40 (2011) 4649-4707. |
| [31] | D.M. Badgujar, M.B. Talawar, S.N. Asthana, et al., Microwave assisted facile synthesis of {1/1,3-bis/1,3,5-tris-[(2-nitroxyethylnitramino)-2,4,6-trinitrobenzene]} using bismuth nitrate pentahydrate as an eco-friendly nitrating agent, J. Hazard. Mater. 152 (2008) 820-825. |
| [32] | D.M. Badgujar, M.B. Talawar, S.N. Asthana, P.P. Mahulikar, Synthesis and characterization of methyl nitramino-2,4,6-trinitobenzenes using bismuth nitrate pentahydrate as an eco-friendly nitrating agent, J. Sci. Ind. Res. 69 (2010) 208-210. |
| [33] | M.M. Khodaei, A.R. Khosropour, M. Beygzadeh, An efficient and environmentally friendly method for synthesis of 3,4-Dihydropyrimidin-2(1H)-ones catalyzed by Bi(NO3)3·5H2O, Synth. Commun. 34 (2004) 1551-1557. |
| [34] | S. Cunha, B.R. de Lima, A.R. de Souza, Bismuth nitrate pentahydrate: a new and environmentally benign reagent for guanidylation of N-benzoylthioureas, Tetrahedron Lett. 43 (2002) 49-52. |
| [35] | V.M. Alexander, R.P. Bhat, S.D. Samant, Bismuth (III) nitrate pentahydrate a mild and inexpensive reagent for synthesis of coumarins under mild conditions, Tetrahedron Lett. 46 (2005) 6957-6959. |
| [36] | B.K. Banik, I. Banik, M. Renteriaa, S.K. Dasgupta, A straightforward highly efficient Paal-Knorr synthesis of pyrroles, Tetrahedron Lett. 46 (2005) 2643-2645. |
| [37] | D.H. Aggen, J.N. Arnold, P.D. Hayes, N.J. Smoter, R.S. Mohan, Bismuth compounds in organic synthesis. Bismuth nitrate catalyzed chemoselective synthesis of acylals from aromatic aldehydes, Tetrahedron 60 (2004) 3675-3679. |
| [38] | H.R. Shaterian, N. Fahimi, K. Azizi, Selective synthesis of 2-aryl-1-benzylated-1Hbenzimidazoles, Chin. J. Chem. 29 (2011) 2389-2393. |
| [39] | J.S. Yadav, B.V. Subba Reddy, K. Premalatha, K. Shiva Shankar, Bismuth(III)-catalyzed rapid and highly efficient synthesis of 2-aryl-1-arylmethyl-1H benzimidazoles in water, Can. J. Chem. 86 (2008) 124-128. |