Lignin is the most abundant biopolymer containing aromatic compound,which is consist of phenylpropane linked by β-O-4, α-O-4,β-5,5-5,4-O-5 and β-β bonds [1]. Increasing attentions have been paid to its potential use as a renewable raw material. However,compared to other biomasses,the utilization of lignin is limited due to its complicated chemical structure and the corresponding chemical reaction mechanism. Therefore,lignin model compound used to address the characteristics of lignin is considered to be the key to the efficient utilization of lignin.
Up to now,a lot of dimeric lignin model compounds have been reported to apply for investigating the characteristics of lignin [2, 3]. Although the synthesis of trimmers [4],tetramers [5], hexamers [6] and oligomers with higher molecular weight [7] are reported,these lignin models are mostly composed of only β-O-4 linkage or two bond linkages which cannot exactly mimic the characteristics of lignin macromolecule. Therefore,it is of great significance to synthesize the models containing a variety of other linkages. Shiba et al. [8] prepared a tetramer lignin model compound composed of 5-5,β-5 and α-O-4 linkages with a yield of 2 mol% (equal to mass percent of 8.0%) catalyzed by laccase at 30 °C for 24 h. Considering the limitation of prolonged time and low yield,a radical synthesis of the tetramer phenolic ligninmodel compound with hydrogen peroxide/horseradish peroxidase and S2O8 2-/Fe2+ as the initiator is investigated in the present work to shorten reaction time and improve yield.
2. Experimental 2.1. Materials and methodsIsoeugenol,ferrous sulfate heptahydrate,potassium persulfate and acetone were purchased from Aladdin Co.,China. All reagents in this work were of analytical grade. High resolution mass spectrum(HRMS)measurementwas carried out on amaXis impact (Bruker,Germany). 1 H NMR and 13 C NMR spectra were recorded with a Bruker DRX-400 spectrometer (Bruker,Germany) with dimethyl sulfoxide (DMSO) as solvent.
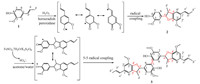
2.2. Synthesis of lignin model compound4-(2,3-Dihydro-7-methoxy-3-methyl-5-propenyl-2-benzofura- nyl)-2-methoxyphenol (2): Isoeugenol (2.4630 g),phosphate buffer solution (60mL,pH 5.83),acetone (45mL) and 10 g/L horseradish peroxidase (4.5mL) were added into a flask equipped with a magnetic stirrer,and then 30% hydrogen peroxide (1.8700 g) was dropwise added. After reaction at 50 °C for 2 h,the crude product was extracted with ethyl acetate and dried under vacuum. The resulting product was further purified by column chromatography with silica gel using dichloromethane/n-hexane (1/5,v/v) as the mobile phase. The purified compound 2 was obtainedwith the yield of 95.3% after evaporation under vacuum. 1 H NMR (400MHz, DMSO-d6): δ 1.32 (d,3H,J = 8.0 Hz,γ-CH3),1.83 (d,3H,J = 4.0 Hz, γ’-CH3),3.81 (s,6H,2 × OCH3),3.41 (m,1H,Hβ),5.11 (d,1H, J =8.0Hz,Hα),6.16 (m,1H,Hβ’ ),6.35 (d,1H,J = 12.0 Hz,Hα’ ), 6.87-7.12 (m,5H,aromatics),9.06 (s,1H,OH); HRMS calculated for C20H22O4 [M+H] + 327.1591,found 327.1599.
5,5' -Bis[2,3-dihydro-7-methoxy-3-methyl-5-propenyl-2-benzo- furanyl]-3,3' -dimethoxy-[1,1' -biphenyl]-2,2' -diol (3): compound 2 (0.3260 g),ferrous sulfate heptahydrate (0.0140 g) and potassium persulfate (0.1360 g) were added into a flask equipped with a magnetic stirrer,then deionized water (10mL) and acetone (20mL) were added to make the solid dissolved. The mixture was stirred at 50-100 °C for 5-25 min. The target product 3 was purified with the same procedure as compound 2. 1 H NMR (400MHz,DMSO-d6): δ 1.31 (d,6H,J = 8.0 Hz,γ-CH3),1.81 (d,6H,J = 4.0 Hz,γ’-CH3),3.78 (s,6H,OCH3),3.82 (s,6H,OCH3),3.41 (m,2H,Hβ),5.09 (d,2H, J =12.0Hz,Hα),6.14 (m,2H,Hβ’ ),6.34 (d,2H,J = 12.0 Hz,Hα’ ), 6.83-6.99 (m,8H,aromatics),8.52 (s,2H,OH); HRMS calculated for C40H42O8Na [M+Na] + 673.2772,found 673.2778. The synthetic route is presented in Scheme 1.
|
Download:
|
| Scheme.1 Synthetic route for phenolic model compound. | |
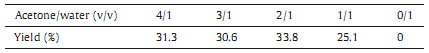
The activation of potassium persulfate promoted by ferrous sulfate to generate sulfate radical is commonly carried out in aqueous media [9]. However,the addition of acetone increases the yield of model compound 3 (Table 1),which shows that a higher yield is obtained when the volume ratio of acetone to water is 2:1. This is because dimericmodel compound 2 is water-insoluble,and an appropriate ratio of acetone to water can make both inorganic initiator and compound 2 dissolve in the reaction media to a large degree. This can be demonstrated from Table 1 that the yield of compound 3 is zero when using water as the solvent.
The influence of temperature on the yield is shown in Table 2. It is found that the yield of model compound increases with the increase of temperature when temperature is less than 80 °C, whereaswhen temperature exceeds 80 °C,the yield decreaseswith the increase of temperature. It is well known that a higher temperature is favorable for generating sulfate radical and therefore increasing the yield of model compound. However, excessive temperature should producemore sulfate radicalswhich can be destructed by the fast reaction with ferrous ion [10], resulting in the decrease of the yield.
| Table 1 Effect of the ratio of solvent on the yield of compound 3. |
| Table 2 The influence of temperature and time on the yield of compound 3. |
HRMS spectrum of compound 3 is shown in Fig. 1a. It shows that the m/z of the target product is 673.2772,which is the adduct mass of model compound 3 with sodium ion. This means that the molecular weight of the synthesized model compound is 650 Da, which is equal to that of C40H42O8. 13 C NMR spectroscopy is a powerful tool to elucidate complicated lignin structures,and the peak of each carbon from 13 C NMR in lignin model compound is labeled in Fig. 1b,in which the peaks at δ 44.54,δ 92.55 and δ 125.34 are assigned to Cβ in β-5,Cα in α-O-4,and C5 in 5-5, respectively.

|
Download:
|
| Fig. 1 HRMS (a) and 13 C NMR (b) spectrum of lignin model compound 3. | |
A tetramer phenolic lignin model compound was prepared by a two-step free radical reactionwith hydrogen peroxide/horseradish peroxidase and S2O8 2-/Fe2+ as the initiator. Compared with enzymatic process,this synthetic process gives a higher yield of 33.8% within a shorter time. HRMS and 13 C NMR spectroscopy show that synthesized model compound contains phenylpropane structure linked by 5-5,α-O-4 and β-5 bonds,which can mimic some chemical characteristics of lignin.
AcknowledgmentsThis work is financially supported by the National Basic Research Program of China (No. 2012CB215302) and the Major Program of National Natural Science Foundation of China (No. 21376100).
| [1] | W.G. Forsythe, M.D. Garrett, C. Hardacre, M. Nieuwenhuyzen, G.N. Sheldrake, An efficient and flexible synthesis of model lignin oligomers, Green Chem. 15 (2013) 3031-3038. |
| [2] | J. Ralph, M.T. Garcia, G. Williamson, Simple preparation of 8-5-coupled diferulate, J. Agric. Food Chem. 46 (1998) 2531-2532. |
| [3] | A. Wu, B.O. Patrick, E. Chung, B.R. James, Hydrogenolysis of β-O-4 lignin model dimers by a ruthenium-xantphos catalyst, Dalton Trans. 41 (2012) 11093-11106. |
| [4] | V.L. Alves, M.G. Drumond, G.M. Stefani, C.L. Chen, D. Pilo-Veloso, Synthesis of new trimeric lignin model compounds containing 5-5' and β-O-4' substructures, and their characterization by 1D and 2D NMR Techniques, J. Brazil. Chem. Soc. 11 (2000) 467-473. |
| [5] | F. Xi, D.W. Reeve, A.B. McKague, The synthesis and chemical structures of tetrameric lignin model compounds, J. Wood Chem. Technol. 16 (1996) 35-46. |
| [6] | I. Kilpeläinen, A. Tervilä-Wilo, H. Peräkylä , J. Matikainen, G. Brunow, Synthesis of hexameric lignin model compounds, Holzforschung 48 (1994) 381-386. |
| [7] | S. Chu, A.V. Subrahmanyam, G.W. Huber, The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound, Green Chem. 15 (2013) 125-136. |
| [8] | T. Shiba, L. Xiao, T. Miyakoshi, C.L. Chen, Oxidation of isoeugenol and coniferyl alcohol catalyzed by laccases isolated from Rhus vernicifera Stokes and Pycnoporus coccineus, J. Mol. Catal. B: Enzym. 10 (2000) 605-615. |
| [9] | M.S. Dasari, K.M. Richards, M.L. Alt, et al., Synthesis of diapocynin, J. Chem. Educ. 85 (2008) 411-412. |
| [10] | C.J. Liang, C.J. Bruell, M.C. Marley, K.L. Sperry, Persulfate oxidation for in situ remediation of TCE. I. Activated by ferrous ion with and without a persulfate-thiosulfate redox couple, Chemosphere 55 (2004) 1213-1223. |