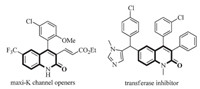
The scaffold of quinolin-2(1H)-one is an outstanding structural motif found inmany natural products and pharmaceutically active compounds [1]. In particular,the derivatives of 4-arylquinolinones have attracted considerable attention in organic chemistry due to their anticancer,antiviral,antibiotic and other activities [2]. Many analogs of this type of heterocyclic compounds have been developed as the lead compounds or clinical candidates [3]. Thus, the synthesis of these valuable compounds has attracted a great deal of interest (Fig. 1).
|
Download:
|
| Fig. 1 Some representative compounds containing the 4-arylquinolinone. | |
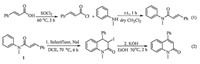
Many strategies have been used in the synthesis of quinolin- 2(1H)-one derivatives including classic base-catalyzed Friedla ¨nder condensation or acid-catalyzed Knorr and Baylis-Hillman reac- tions [4],palladium-catalyzed carbonylative annulation of alkynes with 2-iodoanilines and CO [5],metal-catalyzed carbonylative annulation of internal alkynes [6],palladium-catalyzed tandem cyclization of 2-bromocinnamami-des and aryl iodides [7], Ir-catalyzed annulation of N-arylcarba-moyl chlorides with inter- nal alkynes [8]. However,some of these procedures needed strong acids and the others were carried out in the presence of noble metals. Nonmetal-catalyzed syntheses of 4-aryl-2-quinolinones remain rare. During our previous work for the synthesis of 2-quinolin-2(1H)-one [9],we focused on silver-catalyzed radical tandem cyclization reactions. Herein,we wish to report a semi- one-pot synthesis of 4-arylquinolinones (Scheme 1).
|
Download:
|
| Scheme.1 Semi-one-pot synthesis of 4-arylquinolinones. | |
All reagents were used directly as obtained commercially or after purification. Column chromatography was performed using silica gel (300-400 mesh) and analytical TLC used silica 60-F24. 1 H NMR and 13 C NMR spectra were collected in CDCl3 on a Bruker Fourier 400 MHz spectrometer and chemical shifts (δ) were reported relative to the internal TMS. The substrate 1 was prepared through a short sequence (Scheme 1). A 50 mL anhydrous flaskwas charged with magnetic stir bar,cinnamic acid (5 mmol) and SOCl2 (5 mL). After stirring at 60 °C for 3 h,the redundant SOCl2 was evaporated under reduced pressure and then the liquid was dropwise added into another flask containing N-methylaniline (10 mmol) in anhydrous CH2Cl2 (20 mL). The mixture was stirred for 1 h at room temperature. The organic phase was then washed by aqueous HCl and aqueous K2CO3,then dried over anhydrous Na2SO4. After evaporating the CH2Cl2,the N-methyl-N-phenylcin- namamide was obtained as a pale yellow solid in 97% yield. The yield is almost quantitative and we used it without further purifications.
Substrate 1: Pale yellowsolid, 1 H NMR (400 MHz,CDCl3): δ 7.70 (d,1H,J = 16.0 Hz),7.45 (dt,2H,J = 6.4,1.2 Hz),7.36-7.38 (m,1H), 7.22-7.32 (m,7H,overlapping CDCl3),6.39 (d,1H,J = 16.0 Hz),3.42 (s,3H). 13 C NMR (100 MHz,CDCl3): δ 166.17,143.66,141.70,135.22,129.63,129.49,128.68,127.85,127.59,127.35,118.76, 37.58.
3. Results and discussionN-Methyl-N-phenylcinnamamide 1 was selected for screening the optimal reaction conditions (Scheme 1,Eq. 2).
The substrate 1 and 1.0 equiv. of NaI were added to a flask, then 2.0 equiv. of selectfluor and 5 mL of CH3CN were added,the reaction proceeded at 70 °C for 6 h,after that,the solvent was evaporated and 1.0 equiv. of KOH,3 mL of EtOH were added to the residue,themixture was subsequently stirred at 70 °C for 2h, the desired product was obtained in 35% yield (Table 1,entry 1). Changing the solvent to dichloroethane (DCE),the desired product could be obtained in 55% yield after two steps (Table 1,entry 5). However,other solvents such as dioxane, acetone and CH2Cl2 were not favorable for this transformation (Table 1,entries 2-4). Different oxidants such as DTBP,K2S2O8 and PhI(OAc)2 were also examined under the same conditions (Table 1,entries 9-11),only PhI(OAc)2 showed measurable catalytic effect (Table 1,entry 11). The reaction was also performed at 100 °C,but the results did not improve (Table 1, entry 12). For the step 2,the reaction did not occur using DCE as a solvent (Table 1,entry 13).
| Table 1 Optimization of the reaction. |
After screening the reaction conditions,the substrates testing were carried out subsequently. As shown in Fig. 2,the N-alkyl- N-arylcinnamamides bearing electron-donating or electron- withdrawing groups on the phenyl ring A at the ortho,meta, and para positions are all reactive in the reaction,the corresponding products were obtained in moderate yields (Fig. 2,2-4,12-13). Different substituents such as OMe,Br,Cl, F and Me could be tolerated in the catalytic processes. It is worth noting that halogen atoms (F,Cl,and Br) were well tolerated under the conditions,enabling further functionalization of the corresponding quinolin-2(1H)-ones at the halogenated positions using palladium-catalyzed cross-coupling reactions.

|
Download:
|
| Fig. 2 Structures of synthesized 4-arylquinoline-2(1H)-ones with isolated yield after two steps. | |
In addition,switching the N-protecting group of the substrate to Et or n-Bu,the reaction still proceeded well (Fig. 2,5 and 11). Unfortunately,substituents such as F and Cl at the ortho or para position of aniline (phenyl ring B) affect the efficiency of the reaction dramatically and only a trace amount of products was observed. The NO2 group at the para position of aniline completely shut down the reaction.However,the reaction could proceedwhen a methyl group is at the para position of the aniline and 38% product was obtained (Fig. 2,8).
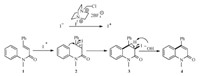
Based on the previous work which described the oxidation of iodide to iodine cation [10],a mechanism for the cyclization/ elimination processes has been proposed (Scheme 2). At first, the iodine anion was oxidized to iodine cation by selectfluor. Then I + is attacked by the electron-rich double bond of N-methyl-N-phenyl-cinnamamide (1) to form intermediate 2. After that,the iodinium ion undergoes nucleophilic attack by phenyl ring to form the six-membered ring. Finally,compound 3 eliminate hydroiodic acid under basic condition to produce compound 4.
|
Download:
|
| Scheme.2 Proposed mechanism for the reaction. | |
In conclusion,we have developed a novel approach for the convenient synthesis of 4-arylquinolin-2(1H)-ones under metal- free conditions. This transformation represents a novel and facile method for the construction of quinolin-2(1H)-one motif without metal catalysis. A mechanism has also been proposed for this transformation.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21302042 and 21172055), the Program for Innovative Research Team from Zhengzhou (No. 131P-CXTD605),and the Plan for Scientific Innovation Talent of Henan University of Technology (No. 171147)
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05.008.
| [1] | (a) B. Joseph, F. Darro, A. Behard, et al., 3-Aryl-2-quinolone derivatives: synthesis and characterization of in vitro and in vivo antitumor effects with emphasis on a new therapeutical target connected with cell migration, J. Med. Chem. 45 (2002) 2543-2555; (b) L. Huang, M. Hsieh, C. Teng, K. Lee, S. Kuo, Synthesis and antiplatelet activity of phenyl quinolones, Biorg. Med. Chem. 6 (1998) 1657-1662; (c) M. Patel, R.J. McHugh, B.C. Cordova, et al., Synthesis and evaluation of quinoxalinones as HIV-1 reverse transcriptase inhibitors, Bioorg. Med. Chem. Lett. 10 (2000) 1729-1731; (d) M. Suzuki, Y. Ohuchi, K.T. Asanuma, et al., Synthesis and evaluation of novel 2-oxo-1,2-dihydro-3-quinolinecarboxamide derivatives as serotonin 5-HT4 receptor agonists, Chem. Pharm. Bull. 48 (2000) 2003-2008. |
| [2] | P. Desos, J.M. Lepagnol, P. Morain, P. Lestage, A.A. Cordi, Structure-activity relationships in a series of 2(1H)-quinolones bearing different acidic function in the 3-position: 6,7-dichloro-2(1H)-oxoquinoline-3-phosphonic acid, a new potent and selective AMPA/kainate antagonist with neuroprotective properties, J. Med. Chem. 39 (1996) 197-206. |
| [3] | (a) T.N. Glasnov, W. Stadlbauer, C.O. Kappe, Microwave-assisted multistep synthesis of functionalized 4-arylquinolin-2(1H)-ones using palladium-catalyzed cross-coupling chemistry, J. Org. Chem. 70 (2005) 3864-3870; (b) P. Hewawasam, W. Fan, J. Knipe, et al., The synthesis and structure-activity relationships of 4-aryl-3-aminoquinolin-2-ones: a new class of calcium-dependent, large conductance, potassium (maxi-K) channel openers targeted for poststroke neuroprotection, Bioorg. Med. Chem. Lett. 12 (2002) 1779-1783. |
| [4] | (a) M. Marull, O. Lefebvre, M. Schlosser, An improved access to 4-trifluoromethyl-2(1H)-quinolinones: the "watering protocol", Eur. J. Org. Chem. 1 (2004) 54-63; (b) B. Palakshi Reddy, P. Iniyavan, S. Sarveswari, V. Vijayakumar, Nickel oxide nanoparticles catalyzed synthesis of poly-substituted quinolines via Friedlander hetero-annulation reaction, Chin. Chem. Lett. 25 (2014) 1595-1600. |
| [5] | D.V. Kadnikov, R.C. Larock, Synthesis of 2-quinolones via palladium-catalyzed carbonylative annulation of internal alkynes by N-substituted o-iodoanilines, J. Org. Chem. 69 (2004) 6772-6780. |
| [6] | (a) D.J. Tang, B.X. Tang, J.H. Li, Selective synthesis of 3-aryl quinolin-2(1H)-ones and 3-(1-arylmethylene)oxindoles involving a 2-fold arene C-H activation process, J. Org. Chem. 74 (2009) 6749-6755; (b) P. Xie, Z.Q. Wang, G.B. Deng, et al., Copper-catalyzed aerobic oxidative carbocyclization-ketonization cascade: selective synthesis of quinolinones, Adv. Synth. Catal. 355 (2013) 2257-2262. |
| [7] | G. Battistuzzi, R. Bernini, S. Cacchi, I. De Salve, G. Fabrizi, 4-Aryl-2-quinolones through a pseudo-domino Heck/Buchwald-Hartwig reaction in a molten tetrabutylammonium acetate/tetrabutylammonium bromide mixture, Adv. Synth. Catal. 349 (2007) 297-302. |
| [8] | T. Iwai, T. Fujihara, J. Terao, Y. Tsuji, Iridium-catalyzed annulation of N-arylcarbamoyl chlorides with internal alkynes, J. Am. Chem. Soc. 132 (2010) 9602-9603. |
| [9] | W.P. Mai, G.C. Sun, J.T. Wang, et al., Silver-catalyzed radical tandem cyclization: an approach to direct synthesis of 3-acyl-4-arylquinolin-2(1H)-ones, J. Org. Chem. 79 (2014) 8094-8102. |
| [10] | L. Shi, D. Zhang, R. Lin, et al., The direct C-H halogenations of indoles, Tetrahedron Lett. 55 (2014) 2243-2245. |





