b Department of Chemistry, Payame Noor University, Tehran 19395-4697, Iran
Multi-component reactions (MCRs) constitute an especially attractive synthetic strategy for rapid and efficient library generation due to the fact that the diversity can be achieved simply by varying the reacting components. To promote the mentioned reactions,various catalytic systems have been used [1, 2, 3]. Recently,the development and application of electrodes as catalyst in organic chemistry,have received considerable attention. Also,electrochemical synthesis as unique technique is valuable for large-scale processes because electricity is a cheap and environmentally responsible chemical reagent and its interest behavior to produce initial base as catalyst can be used at ambient temperature and pressure [4]. According to this information,many chemical transformations such as MCRs to prepare various suitable compounds can be performed.
1,4-Dihydropyrano[2,3-c]pyrazole derivatives are a class of important heterocycles with a wide range of biological and pharmacological properties such as antimicrobial [5],insecticidal and molluscicidal activities [6],anticancer,anticoagulant,diuretic, spasmolytic and antianaphylactic agents [7]. Many methods with different conditions have been reported. Some of these compounds have been already prepared in the presence of CuO-CeO2 [7], triethylammonium acetate (TEAA) [6],imidazole [8],L-proline or KF-alumina [9],Silica bonded n-propyl-4-aza-1-azoniabicyclo[ 2.2.2]octane chloride (SB-DABCO) [10] and H14[NaP5W30O110] [11].
However,some of these protocols require long reaction times, multi-step reactions and complex synthetic pathways and afford products with only modest yields. Therefore,the introduction of milder,faster and more ecofriendly methods,accompanied with higher yields are needed.
In continuation of previous work [12] to develop efficient and environmentally benign procedures,we report the successful synthesis of 4-dihydropyrano[2,3-c]pyrazole derivatives via direct addition of various aromatic aldehydes,malononitrile and 3- methyl-1-phenyl-1H-pyrazol-5(4H)-one in an undivided cell at room temperature under a constant current density and without base or any additive catalyst in green media.
2. Experimental
The structural evaluation studies of compounds were performed with various experimental techniques such as IR,elemental analysis,1H NMR and 13C NMR spectroscopies. Complete relevant spectra (1H NMR,13C NMR and IR) for the products are available in the Supporting information. 2.1. Typical experimental procedure for electrocatalytic synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives
A mixture of aryl aldehyde (2 mmol),malononitrile (3 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (2 mmol),and NaBr (0.05 g,0.5 mmol) in EtOH (20 mL) was electrolyzed in an undivided cell equipped with a magnetic stirrer,a graphite anode, and an iron cathode at 25 °C under a constant current density of 10 mA/cm2 [electrodes square 5 cm2] until the catalytic quantity of 0.62 F/mol of electricity was passed. After the electrolysis was finished,the precipitated products were separated by filtration which was then twice rinsed with an ice-cold ethanol/water solution (9:1,5 mL),and dried under reduced pressure. 2.2. Analytical data for selected compounds
6-Amino-4-(3-ethoxy-4-hydroxyphenyl)-3-methyl-1-phenyl- 1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (4o): Mp: 189- 171 °C; IR (KBr,cm-1): νmax 2195 (C≡N);3329-3420 (NH2),1H NMR (400 MHz,DMSO-d6):δ1.29-1.33 (t,3H,J = 7.2 HzCH3),1.83 (s,3H,CH3),3.96-4 (q,2H,J = 7.2 Hz CH2),4.58 (s,1H,CH),6.60- 7.82 (m,NH2 and Ar),8.85 (s,1H,OH); 13C NMR (100 MHz, DMSO-d6):δ13 (CH3),15 (CH3),36.7 (CH),59 (CN),64 (CH2),99.4, 114,116,120.3,120.6,120.65,126.5,130,135,138. 144,145.9, 146,146.3,146.8,146.9,159.6,159.7; Anal. Calcd. for C22H20N4O3: C,68.03; H,5.19; N,14.42%. Found: C,68.12; H,5.21; N,14.38.
4,4'-(1,4-Phenylene)bis(6-amino-3-methyl-1-phenyl-1,4-dihydropyrano[ 2,3-c]pyrazole-5-carbonitrile) (4p): Mp: 236-238 °C; IR (KBr,cm-1): νmax 2195 (C≡N);3329-3366 (NH2),1H NMR (400 MHz,DMSO-d6):δ1.79 (s,3H,CH3),4.80 (s,1H,CH),7.3 -8.6 (m,NH2 and Ar); 13C NMR (100 MHz,DMSO-d6):δ13 (CH3),34.7 (CH),57.7,98.2,120.4,120.6,124.4,126.7,129.8,136,137.9,139.4, 144.5,145.5,149,149.5,160,160.1,Anal. Calcd. for C34H26N8O2: C, 70.58; H,4.53; N,19.37% Found: C,70.33; H,4.53; N,19.59.
6-Amino-4-(biphenyl-4-yl)-3-methyl-1-phenyl-1,4-dihydropyrano[ 2,3-c]pyrazole-5-carbonitrile (4q): Mp: 193-194 °C; IR (KBr,cm-1): νmax 2195 (C≡N);3328-3471(NH2),1H NMR (400 MHz,DMSO-d6):δ1.77 (s,3H,CH3),4.88 (s,1H,CH),7.31- 7.95 (m,NH2 and Ar); 13CNMR(100 MHz,DMSO-d6):δ13.4 (CH3),37 (CH),58,99,120.4,120.6,126.6,127,127.3,127.8,129,129.8,138, 139,140,143,144,145.8,159.95,160,Anal. Calcd. for C26H20N4O: C, 77.21; H,4.98; N,13.85%. Found: C,77.14; H,4.99; N,14.01.
3. Results and discussionOur investigations on the electrocatalytic multicomponent chain transformation of aryl aldehydes,3-methyl-1-phenyl-1Hpyrazol- 5(4H)-one and malononitrile into 6-amino-3-methyl-1, 4-diphenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles under neutral and mild conditions by electrolysis in an undivided cell, began with the optimization of the reaction conditions. The synthetic pathway is shown in Scheme 1.

|
Download:
|
| Scheme.1 .Synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives catalyzed by an electrogenerated base. | |
Table 1 illustrates the obtained data for the synthesis of 6- amino-3-methyl-1,4-diphenyl-1,4-dihydropyrano[2,3-c]pyrazole- 5-carbonitrile 4a under various experimental conditions. The reaction is performed in alcohols or acetonitrile as solvent in the presence of an electrolyte at room temperature. The reaction progress was monitored with TLC.
| Table 1 Optimization of reaction conditions for synthesis of 6-amino-3-methyl-1,4-diphenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile at room temperature.a |
Various amounts of current were applied under the mentioned conditions. Excellent conversions of the starting materials were obtained under 10 mA/cm2 current densities after 0.48 F/mol of electricity had been passed and I = 50 mA,electrodes surface (5 cm2) was found to be optimal for the electrochemically induced chain process and allowed for the highest yield of 4a in solvent of CH3CN and electrolyte of tetrabutyl ammonium bromide.
As seen in Table 1,acetonitrile as solvent can be useful and the related yield is excellent especially when combined with tetrabutylammonium bromide (Bu4NBr) but this solvent is more expensive rather than an alcohol such as ethanol. Also,Acetonitrile has a modest toxicity in small doses. It can be metabolized to produce hydrogen cyanide,which is the source of the observed toxic effects [13, 14]. Meanwhile,Bu4NBr commonly used as a phase transfer catalyst. Weapplied this compound as electrolyte in acetonitrile and ethanol. The results were shown Bu4NBr can be effective and the product was formed in excellent yield even when EtOH was used as solvent. However,as mentioned in acetonitrile, Bu4NBr is more expensive and very hazardous.
Under these conditions,conversions of the starting materials were allowed for the excellent yield of 4a in ethanol as a solvent and sodium bromide was used as an electrolyte.
An increase in the current density up to 15 mA/cm2 (I = 75 mA) resulted in a slight decrease in the reaction yield,and may be a result of the activation of the undesired direct electrochemical processes that lead to oligomerization of the starting material.
In comparison of electrocatalytic method with chemical method,we used sodium metal as catalyst (10 mol%) for preparation of 4a,but desired product was obtained in low yields. So it seem this method has not preferable for certainly economical advantages. Regarding to Table 1,electrocatalytic method in comparison with other chemical methods has some advantages that it is green and environmentally friendship and catalyst is produced and consumed in reaction media.
Using the optimized conditions,we also probed the scope and generality of the reaction of several aryl aldehydes with malononitrile and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one for synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives (Table 2,4a-q).
| Table 2 Electrocatalytic multicomponent synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivative under optimized conditions.a |
As shown in Table 2,products were obtained in excellent yields. It was found that aromatic aldehydes both with electron withdrawing and donating groups in reaction with other starting material have excellent isolated yield. Notably,in examining their synthetic performance,it has been shown that this method is capable of promoting organic synthesis of 1,4-dihydropyrano[ 2,3-c]pyrazole derivatives in an environmentally friendly condition. A catalytic amount of an electrogenerated base can efficiently induce the catalytic chain transformation of organic compounds. All these qualities agree well with the rules of green chemistry [4].
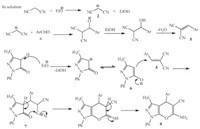
The proposed mechanism for the preparation of related products is depicted in Scheme 2. The initial formation of bromine on the anode is a well-known process and the corresponding halogen color was observed at the anode when the electrolysis was conducted without stirring the reaction mixture [10]. Deprotonation of an alcohol at the cathode leads to formation of alkoxide anion [yes]. Its subsequent reaction in solution with malononitrile gives rise to malononitrile anion.
|
Download:
|
| Scheme. 2.The proposed mechanism for the formation of 1,4-dihydropyrano[2,3- c]pyrazole derivatives. | |
Then Knoevenagel condensation of aldehyde with malononitrile anion takes place in the solution with the elimination of water and formation of the corresponding a-cyanocinnamonitrile derivatives 5.
We perform a control experiment to detect of intermediate 5. The reaction was take placed in presence of benzaldehyde and malononitrile at mentioned conditions (room temperature,I = 50 mA,EtOH and NaBr) and the intermediate 5 was formed at 5 min in excellent yield (96%). Michael addition between 5 and 6 furnished 7,which upon intramolecular cyclization and isomerization gave rise to product 4.
4. Conclusion
In conclusion,we have developed an efficient green procedure for the one-pot synthesis of 1,4-dihydropyrano[2,3-c]pyrazole derivatives in excellent yields under neutral and mild conditions in the presence of sodium bromide as an electrolyte. The very short reaction time,high yields,simple workup the non-chromatographic purification of products will make the present method an important addition to the available methodologies for synthesis of the products.
Acknowledgment
We are thankful to University of Sistan and baluchestan and Payame Noor University for partial support of this work. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.016.
| [1] | R.C. Cioc, E. Ruijter, R.V.A. Orru, Multicomponent reactions: advanced tools for sustainable organic synthesis, Green Chem. 16 (2014) 2958-2975. |
| [2] | L.A. Wessjohann, R.A.W. Neves Filho, A.R. Puentes, M.C. Morejon, Macrocycles from multicomponent reactions, multicomponent reactions in organic synthesis, vol. 2, Wiley-VCH Verlag GmbH & Co. KGaA, 2015, pp. 231-261. |
| [3] | S. Su, C. Li, X. Jia, J. Li, Isocyanide-based multicomponent reactions: concise synthesis of spirocyclic oxindoles with molecular complexity by using a [1,5]-hydrogen shift as the key step, Chemistry 20 (2014) 5905-5909. |
| [4] | L.Wang, J. Gao, L.Wan, Y.Wang, C. Yao, Electrocatalytic multicomponent transformation of cyclopentane-1,3-dione, aldehydes andmalononitrile: an efficientway to cyclopenta[b]pyran derivatives, Res. Chem. Intermed. 41 (2015) 2775-2784. |
| [5] | A. Shaabani, A. Sarvary, A.H. Rezayan, S. Keshipour, Synthesis of fully substituted pyrano[2,3-c]pyrazole derivatives via a multicomponent reaction of isocyanides, Tetrahedron 65 (2009) 3492-3495. |
| [6] | R.S. Balaskar, S.N. Gavade, M.S. Mane, et al., Greener approach towards the facile synthesis of 1, 4-dihydropyrano [2,3-c] pyrazol-5-yl cyanide derivatives at room temperature, Chin. Chem. Lett. 21 (2010) 1175-1179. |
| [7] | J. Albadi, A. Mansournezhad, Z. Derakhshandeh, CuO-CeO2 nanocomposite: a highly efficient recyclable catalyst for the multicomponent synthesis of 4Hbenzo[ b]pyran derivatives, Chin. Chem. Lett. 24 (2013) 821-824. |
| [8] | A. Siddekhab, A. Nizama, M.A. Pasha, An efficient and simple approach for the synthesis of pyranopyrazoles using imidazole (catalytic) in aqueous medium, and the vibrational spectroscopic studies on 6-amino-4-(4'-methoxyphenyl)-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole using density functional theory, Spectrochim. Acta A 81 (2011) 431-440. |
| [9] | H.Mecadon, R. Rohman, I. Kharbangar, et al., L-Proline as an efficicent catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in water, Tetrahedron Lett. 52 (2011) 3228-3231. |
| [10] | A. Hasaninejad, M. Shekouhy, N. Golzara, A.K. Zareb, M.M. Doroodmand, Silica bonded n-propyl-4-aza-1-azoniabicyclo[2.2.2]octane chloride (SB-DABCO): A highly efficient, reusable and new heterogeneous catalyst for the synthesis of 4H-benzo[b]pyran derivatives, Appl. Catal. A: Gen. 402 (2011) 11-22. |
| [11] | M.M. Heravi, A. Ghodsa, F. Derikvand, K. Bakhtiari, F.F. Bamoharram, H14[NaP5W30O110] catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivatives, J. Iran. Chem. Soc. 7 (2010) 615-620. |
| [12] | Z. Vafajoo, H. Veisi, M.T. Maghsoodlou, H. Ahmadian, Electrocatalytic multicomponent assembling of aldehydes, 4-hydroxycoumarin and malononitrile: an efficient approach to 2-amino-5-oxo-4,5-dihydropyrano(3,2-c)chromene-3-carbonitrile derivatives, C. R. Chim. 17 (2014) 301-304. |
| [13] | P. Wexler, Encyclopedia of Toxicology, second ed., Elsevier, 2005. |
| [14] | M. Greenberg, in: U.S. Environmental Protection Agency, Washington, DC, 1999, pp. 35. |
| [15] | S.R. Mandha, S. Siliveri, M. Alla, V.R. Bommena, M.R. Bommineni, S. Balasubramanian, Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c] pyrazoles, Bioorg. Med. Chem. Lett. 22 (2012) 5272-5278. |
| [16] | C.S. Yao, Y. Wang, T.J. Li, et al., A pseudo multi-component electrochemical synthesis of spiro dihydrofuran derivatives, Tetrahedron 69 (2013) 10593-10597. |
| [17] | M.N. Elinson, A.I. Ilovaisky, A.S. Dorofeev, et al., Electrocatalytic multicomponent transformation of cyclic 1,3-diketones, isatins, and malononitrile: facile and convenient way to functionalized spirocyclic (5,6,7,8-tetrahydro-4H-chromene)-4,3'-oxindole system, Tetrahedron 63 (2007) 10543-10548. |
| [18] | S. Makarem, A.A. Mohammadi, A.R. Fakhari, A multi-component electro-organic synthesis of 2-amino-4H-chromenes, Tetrahedron Lett. 49 (2008) 7194-7196. |
| [19] | M.N. Elinson, A.S. Dorofeev, R.F. Nasybullin, G.I. Nikishin, Facile and convenient synthesis of 4,4'-(Arylmethylene)bis(1H-pyrazol-5-ols) by electrocatalytic Tandem Knoevenagel-Michael reaction, Synthesis (2008) 1933-1937. |