C-H functionalization approach for its economic and environmentally benign features in organic synthesis has been the research hotspot for many years [1] and has emerged as one of the most efficient strategies for the construction of C-C [2] and C-X [3] bonds. In particular,various cross-dehydrogenative-coupling (CDC) reactions to form C-N bonds have also been developed greatly [4] as the product can usually be used in the synthesis of bioactive nitrogen-containing compounds.
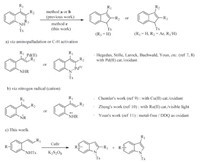
Nitrogen-containing heterocycles,especially indole derivatives, have attracted more attentions as important structural motifs in a variety of biologically active natural products [5]. In the past decade,numerous methods for the construction of indole moieties and their derivatives have been developed [6]. Among them,C-H amination reactions of 2-alkenyl- or 2-alkynylaniline are often used to form an indole moiety. Since Hegedus and co-workers reported the synthesis of nitrogen heterocycles though palladiumassisted intramolecular amination of olefins in 1978 [7],Pd(II)- catalyzed aminopalladation has emerged as one of the most efficient methods to the synthesis of indoles (Scheme 1a) [8]. However,a radical mechanism has also been implicated in the reactions of the same substrates (Scheme 1b). The Chemler group reported a Cu-catalyzed intramolecular oxidative amination of alkenes for the synthesis of indoles,presenting a mechanism involving nitrogen-radical addition to the alkenes [9]. The Zheng group reported a photocatalytic synthesis of indoles also involving a nitrogen-centered radical cation [10]. Very recently,the Youn group reported a metal-free C-H amination of N-Ts-2-Styrylaniline by using DDQ as an oxidant to form the nitrogen-radical cation [11]. In consideration of the widely existence of indoles in bioactive substance and pharmaceuticals,we wanted to expand the synthetic path of indoles. As our group has concentrated on the study of the transition metal catalyzed C-H activation reactions [12],herein we report the C-H amination of 2-alkenyl- or 2- alkynylaniline to construct indoles moiety through an easy and simple procedure with no expensive transition metal.
|
Download:
|
| Scheme. 1.Indole synthesis from 2-alkenylanilines. | |
All reagents and solvents were analytical grade and purchased from commercial sources,used without further purification,if not otherwise stated. Merck 60 silica gel was used for chromatography, and Whatman silica gel plates with fluorescence F254 were used for thin-layer chromatography (TLC) analysis. 1H NMR and 13C NMR spectra were recorded on Bruker Avance 400,and tetramethylsilane (TMS) or CDCl3 (7.26 ppm for 1H NMR,77.0 ppm for 13C NMR) was used as a reference. Data for 13C NMR were reported as ppm. High resolution mass spectra (HRMS) were performed on a Waters Micromass GCT instrument.
General procedure for indole products: To a Schlenk pressure tube,CuBr (0.025 mmol),N-Ts-2-Styrylaniline (1) (0.25 mmol) and K2S2O8 (1.25 mmol) were placed. Then 2 mL MeCN was added. The tube was sealed and flushed with nitrogen,and then the contents were stirred at 110 °C for 24 h. The reaction mixture was cooled to room temperature and poured into water and then the product was extracted with CH2Cl2 (3 times). The combined organic layer was washed with brine,dried over MgSO4,and concentrated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether:EtOAc = 10:1).
3. Results and discussion
Firstly N-Ts-2-Styrylaniline (1a) was chosen as standard substrate to find the optimized reaction conditions and the results were summarized in Table 1. Based on our previous research,the FeCl3/t-BuOOH system was firstly applied to this reaction,however no desired product was detected (Table 1,entries 1-2). Then Pd(OAc)2 was used as the catalyst and acetonitrile (MeCN) was used as a solvent at 60 °C in air and under an oxygen atmosphere, the product was found only in 21% (Table 1,entries 3-4). Thinking of the expensive price and its toxicity,Pd(OAc)2 was replaced by CuBr,and when CuBr was used as the catalyst,the desired product 2a was isolated only in 11% yield after 24 h at 60 °C (Table 1,entry 5). It was unsuitable to increase the reaction temperature (Table 1, entry 6). Considering the K2S2O8 was usually used to provided radical particles [13],it was applied to the reaction at 110 °C under a nitrogen atmosphere then a good yield of 2a was received (Table 1,entry 7). When the amount of K2S2O8 was reduced,the yield of product 2a was also reduced (Table 1,entries 8-9). Then, other frequently used copper (I) salts were screened (Table 1, entries 10-11),and CuBr was the preferable choice in this reaction. When CuBr was removed and only K2S2O8 was added to the reaction system,2a and 2a' were collected at the ratio of 1:10 with a total yield of 55% (Table 1,entry 12).
| Table 1 Optimization of the reaction conditions.a |
With the optimized reaction conditions established (Table 1, entry 7),the generality of the reaction was explored. The results were outlined in Table 2. Firstly the effects of substituents (R) residing on the aromatic part of N-Ts-2-Styrylaniline were screened. As we can see both electron-withdrawing and electron- donating substituents worked well under the standard reaction conditions to afford the corresponding indoles in good to excellent yields (Table 2,entries 1-8),whereas the low yield of methyl group (Table 2,entries 6,14 and 17) was attributed to the easy oxidation of the benzylic position. When there was orthomethyl on the aromatic part,no desired product was detected (Table 2,entries 9-10). Subsequently the substituent effect at the alkene part was also explored (Table 2,entries 12-16). Aliphatic alkenes such as n-octylene and n-amylene were also suitable to this system. Noteworthy was that when 1n was applied in this reaction,a mixture of 2- and 3-substituted indole products (2n:2n0 = 0.7:10) were collected which indicated a migratorial process occurred (Table 2,entry 17) [14]. N-Ts-2-Styrylnaphthylamine and N-Ts-4-chloro-2-cinnamylaniline were applied to this reaction,however no corresponding product was got (Table 2, entries 11 and 18).
| Table 2 Scope of the reaction.a |
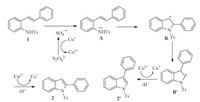
Further experiment was conducted in order to understand the mechanism of this reaction,2,2,6,6-tetramethylpiperdine loxyl (TEMPO),a well-known radical-trapping reagent,was added to the reaction,while the trapping product was not obtained,and the corresponding 2-phenyl-1-tosyl-1H-indole (2a') was collected in the yield of 83% together with little starting material. According to the experiment and literature [11,13,14a],a plausible mechanism was provided in Scheme 2. The sulfate radical could be produced by the single electron transfer from Cu+ to S2O8 2- when thermal energy is applied. Then radical cation intermediate A might be formed by a dehydrogenation of 1 facilitated by sulfate radical anion. The nitrogen radical cation could add to the double bond of the alkene,generating intermediate B which then rearranged to B'. Under oxidizing conditions,B and B' were oxidized to give corresponding C-H amination products.
|
Download:
|
| Scheme. 2.Proposed mechanism. | |
In summary,we have successfully realized the direct intramolecular C-H amination reactions of N-Ts-2-Styrylaniline derivatives catalyzed by copper and potassium peroxydisulfate to get indole derivatives under mild conditions. Further investigations of the application in organic reactions are underway in our laboratory.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 21272169,21302135),the Key Innovation Team of Science & Technology in Zhejiang Province (No. 2010R50018-10),the Science and Technology Bureau of Taizhou (No. 08KY10) and the Key Disciplines of Applied Chemistry of Zhejiang Province,Taizhou University.
| [1] | (a) S.W. Youn, S.J. Pastine, D. Sames, Ru(III)-catalyzed cyclization of arene-alkene substrates via intramolecular electrophilic hydroarylation, Org. Lett. 6 (2004) 581-584; (b) X.D. Zhao, E. Dimitrijević, Vy M. Dong, Palladium-catalyzed C-H bond functionalization with arylsulfonyl chlorides, J. Am. Chem. Soc. 131 (2009) 3466-3467; (c) S. Pan, J. Liu, H. Li, et al., Iron-catalyzed N-alkylation of azoles via oxidation of C-H bond adjacent to an oxygen atom, Org. Lett. 12 (2010) 1932-1935; (d) X. Wang, L. Truesdale, J.Q. Yu, Pd(II)-catalyzed ortho-trifluoromethylation of arenes using TFA as a promoter, J. Am. Chem. Soc. 132 (2010) 3648-3649; (e) P.Y. Choy, C.P. Lau, F.Y. Kwong, Palladium-catalyzed direct and regioselective C-H bond functionalization/oxidative acetoxylation of indoles, J. Org. Chem. 76 (2011) 80-84; (f) H. Xu, Y. Zhang, J. Huang, W.Z. Chen, Copper-catalyzed synthesis of N-fused heterocycles through regioselective 1,2-aminothiolation of 1,1-dibromoalkenes, Org. Lett. 12 (2010) 3704-3707. |
| [2] | For selected examples: (a) T Nishikata, A.R. Abela, S. Huang, B.H. Lipshutz, Cationic palladium(II) catalysis: C-H activation/Suzuki-Miyaura couplings at room temperature, J. Am. Chem. Soc. 132 (2010) 4978-4979; (b) A.J.A. Watson, A.C. Maxwell, J.M.J. Williams, Ruthenium-catalyzed aromatic C-H activation of benzylic alcohols via remote electronic activation, Org. Lett. 12 (2010) 3856-3859; (c) H.A. Duong, R.E. Gilligan, M.L. Cooke, M.J. Gaunt, Copper(II)-catalyzed metaselective direct arylation of a-aryl carbonyl compounds, Angew. Chem. Int. Ed. 49 (2010) 1-5; (d) C.X. Song, G.X. Cai, T.R. Farrell, Z.J. Shi, Direct functionalization of benzylic C-Hs with vinyl acetates via Fe-catalysis, Chem. Commun. (2009) 6002-6004; (e) D.J. Tang, B.X. Tang, J.H. Li, Selective synthesis of 3-aryl quinolin-2(1H)-ones and 3-(1-arylmethylene)oxindoles involving a 2-fold arene C-H activation process, J. Org. Chem. 74 (2009) 6749-6755; (f) C. Guo, J. Song, S.W. Luo, L.Z. Gong, Enantioselective oxidative cross-coupling reaction of 3-indolylmethyl C-H bonds with 1, 3-dicarbonyls using a chiral Lewis acid-bonded nucleophile to control stereochemistry, Angew. Chem. Int. Ed. 49 (2010) 5558-5562. |
| [3] | For selected examples: (a) A Boyer, N. Isono, S. Lackner, M. Lautens, Domino rhodium(I)-catalysed reactions for the efficient synthesis of substituted benzofurans and indoles, Tetrahedron 66 (2010) 6468-6482; (b) V.A. Schmidt, E.J. Alexanian, Metal-free, aerobic dioxygenation of alkenes using hydroxamic acids, Angew. Chem. Int. Ed. 49 (2010) 1-5; (c) G. Song, D. Chen, R.H. Crabtree, X.W. Li, Rh-catalyzed oxidative coupling between primary and secondary benzamides and alkynes: synthesis of polycyclic amides, J. Org. Chem. 75 (2010) 7487-7490; (d) K. Morimoto, K. Hirano, T. Satoh, M. Miura, Rhodium-catalyzed oxidative coupling/cyclization of 2-phenylindoles with alkynes via C-H and N-H bond cleavages with air as the oxidant, Org. Lett. 12 (2010) 2068-2071; (e) C.P. Frazier, J.R. Engelking, J.R. Alaniz, Copper-catalyzed aerobic oxidation of hydroxamic acids leads to a mild and versatile acylnitroso ene reaction, J. Am. Chem. Soc. 133 (2011) 10430-10433; (f) L. Chu, X. Yue, F.L. Qing, Cu(II)-mediated methylthiolation of aryl C-H bonds with DMSO, Org. Lett. 12 (2010) 1644-1647. |
| [4] | For selected examples: (a) S Li, J. Wu, Synthesis of H-pyrazolo[5,1-a]isoquinolines via copper(II)-catalyzed oxidation of an aliphatic C-H bond of tertiary amine in air,, Org. Lett. 13 (2011) 712-715; (b) H.G. Wang, Y. Wang, D.D. Liang, Q. Zhu, Copper-catalyzed intramolecular dehydrogenative aminooxygenation: direct access to formyl-substituted aromatic N-heterocycles, Angew. Chem. Int. Ed. 50 (2011) 1-5; (c) H.G. Wang, Y. Wang, C.L. Peng, J.C. Zhang, Q. Zhu, A direct intramolecular C-H amination reaction cocatalyzed by copper(II) and iron(III) as part of an efficient route for the synthesis of pyrido[1,2-a]benzimidazoles from N-aryl-2-aminopyridines, J. Am. Chem. Soc. 132 (2010) 13217-13219; (d) S.M. Guo, B. Qian, Y.J. Xie, C.G. Xia, H.M. Huang, Copper-catalyzed oxidative amination of benzoxazoles via C-H and C-N bond activation: a new strategy for using tertiary amines as nitrogen group sources, Org. Lett. 13 (2011) 522-525. |
| [5] | (a) G.W. Gribble, Recent developments in indole ring synthesis-methodology and applications, J. Chem. Soc., Perkin Trans. 1 (2000) 1045-1075; (b) S. Hibino, T. Choshi, Simple indole alkaloids and those with a nonrearranged monoterpenoid unit, Nat. Prod. Rep. 19 (2002) 148-180. |
| [6] | (a) L.S. Hegedus, Transition metals in the synthesis and functionalization of indoles [new synthesis methods (72)], Angew. Chem., Int. Ed. 27 (1988) 1113-1126; (b) G. Zeni, R.C. Larock, Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry, Chem. Rev. 104 (2004) 2285-2310; (c) P. Luo, R.Y. Tang, P. Zhong, J.H. Li, Progress in the synthesis of nitrogencontaining heterocycles by in-tramolecular cyclization of alkynes, Chin. J. Org. Chem. 29 (2009) 1924-1937; (d) D.F. Taber, P.K. Tirunahari, Indole synthesis: a review and proposed classification, Tetrahedron 67 (2011) 7195-7210. |
| [7] | L.S. Hegedus, G.F. Allen, J.J. Bozell, E.L. Waterman, Palladium-assisted intramolecular amination of olefins. synthesis of nitrogen heterocycles, J. Am. Chem. Soc. 100 (1978) 5800-5807. |
| [8] | (a) P.J. Harrington, L.S. Hegedus, Palladium-catalyzed reactions in the synthesis of 3-and 4-substituted indoles. Approaches to ergot alkaloids, J. Org. Chem. 49 (1984) 2657-2662; (b) S. Cacchi, G. Fabrizi, Synthesis and functionalization of indoles through palladium-catalyzed reactions, Chem. Rev. 105 (2005) 2873-2920; (c) G. Zeni, R.C. Larock, Synthesis of heterocycles via palladium-catalyzed oxidative addition, Chem. Rev. 106 (2006) 4644-4680; (d) S.W. Youn, J.H. Bihn, B.S. Kim, Pd-catalyzed intramolecular oxidative C-H amination: synthesis of carbazoles, Org. Lett. 13 (2011) 3738-3741. |
| [9] | T.W. Liwosz, S.R. Chemler, Copper-catalyzed oxidative amination and allylic amination of alkenes, Chem. Eur. J. 19 (2013) 12771-12777. |
| [10] | S. Maity, N. Zheng, A visible-light-mediated oxidative C-N bond formation/ aromatization cascade: photocatalytic preparation of N-arylindoles, Angew. Chem. Int. Ed. 51 (2012) 9562-9566. |
| [11] | Y.H. Jang, S.W. Youn, Metal-free C-H amination for indole synthesis, Org. Lett. 16 (2014) 3720-3723. |
| [12] | (a) J.G. Yang, Z.J. Wang, F.Y. Pan, W.L. Bao, CuBr-catalyzed selective oxidation of N-azomethine: highly efficient synthesis of methine-bridged bis-indole compounds, Org. Biomol. Chem. 8 (2010) 2975-2978; (b) J.G. Yang, D. Chen, W.L. Bao, Stereo-selective synthesis of cis-alkenes/haloalkenes by reaction of diphenylmethane with ethynylbenzenes via sp3 C-H bond activation promoted by iron salts, Tetrahedron Lett. 53 (2012) 3984-3989; (c) D. Chen, F.Y. Pan, J.R. Gao, J.G. Yang, Iron catalyzed direct C(sp3)-H amination reactions of isochroman derivatives with primary arylamines under mild conditions, Synlett 24 (2013) 2085-2088. |
| [13] | Z.Y. Xie, Y.P. Cai, J.L. Jiang, Cu-catalyzed cross-dehydrogenative coupling reactions of (benzo)thiazoles with cyclic ethers, Org. Lett. 15 (2013) 4600-4603. |
| [14] | (a) J. Wang, S. Wang, J. Zhang, X.Q. Yu, ChemInform abstract: iron-mediated direct arylation with arylboronic acids through an aryl radical transfer pathway, Chem. Commun. 48 (2012) 11769-11771; (b) S.A. Shahzad, C. Vivant, T. Wirth, Selenium-mediated synthesis of biaryls through rearrangement, Org. Lett. 12 (2010) 1364-1367; (c) B.J. Stokes, S. Liu, T.G. Driver, Rh2(II)-catalyzed nitro-group migration reactions: selective synthesis of 3-nitroindoles from β-nitro styryl azides, J. Am. Chem. Soc. 133 (2011) 4702-4705. |






