b Department of Physics, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
Dye-sensitized solar cells (DSSCs) have attracted a great deal of attention in recent years owing to their low cost,facile fabrication, flexibility,and potential indoor applications [1, 2]. As a key component of DSSCs,the sensitizers work to absorb the sunlight and convert light into photocurrent [3]. Various photosensitizers have been devised,of which the ruthenium complexes,porphyrin compounds and metal-free organic dyes are mostly studied. The ruthenium and porphyrin based complexes have achieved promising performance [12, 13]. However,the large scale production of these metal complexes is difficult due to the high cost and environmental issues.Onthe contrary,themetal-free organic sensitizershavemany advantages such as low cost,facile synthesis,high molar extinction coefficients and tunable optoelectronic properties by suitable molecular design. Therefore,in recent years more and more attention has been paid to the investigation of organic dye and great achievements have been gained (efficiency approaching 12%) [17].Generally,themost commondesignmotif for a dyemolecule is thedonor and acceptormoieties connectedbyapspacer.TheD-p- A architecture is facilitating for efficient intramolecular charge transfer (ICT),and it therefore allows efficient charge separation and is beneficial for photoelectron injection [18, 19].
Truxene could be considered as 2-dimensinal aromatics with three-fold symmetry. With the rigid p-conjugated structure,it has received considerable attention and shows potential applications in fields like organic light-emitting diodes (OLEDs) and nonlinear optical materials (NLO) [20]. Solution processable pconjugated triphenylamine-based dendrimers with truxene core have served as hole-transporting materials (HTMs) for organic light-emitting device. The dendrimers are thermally stable and exhibited slightly higher maximum current efficiency and lower turn-on voltage compared with the NPB-based device [21, 22]. Branched molecules with truxene core have been used in lasers and field effect transistors [25]. Nevertheless,the truxene structure is rarely investigated for photovoltaic applications [20- 24]. Star-shaped truxene fused with three benzo[2,1-b:3,4- b0]dithiophenes was used as donor material for bulk heterojunction photovoltaic devices. With PC71BM as the acceptor material, the fabricated solar cells exhibited a power conversion efficiency of 2.40% with open circuit voltages in the range of 0.86-1.07 V [26]. Organic sensitizers featuring unsymmetrical truxene-based triarylamine donor were applied for DSSCs. The incorporation of strong electron donor unit resulted in improved light harvesting capacity,photocurrent,as well as the solar cell efficiency. Power conversion efficiency over 7%were achieved employing Co(II/III)- based electrolyte under standard AM 1.5 sunlight [20]. Whereas as a whole,the investigation of functionalized truxene dyes as the photosensitizers in DSSCs have not been well explored yet.
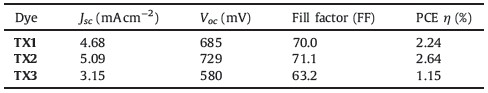
It is well known that the hexaalkyltruxene group could increase the dye solubility,and moreover it could prevent the liquid electrolyte from approaching the nanocrystalline TiO2 film [27]. In addition,the hexyl chains on the truxene core may prevent aggregation between the dye molecules,which is intimately correlated with the ultimate power output of a DSSC. Based on these considerations,we present three new organic sensitizers containing truxene as the core structure,triphenylamine as the electron donor,cyanoacetic acid or rhodanine 3-acetic acid as the electron acceptor and also the anchoring group. In TX1,there are two triphenylamine groups and one cyanoacetic acid group. In TX2,there are two cyanoacetic acid acceptor groups,while only one triphenylamine donor. TX3 has similar structure to TX1 except that the acceptor moiety is changed into rhodanine 3-acetic acid. Their molecular structures are shown in Scheme 1. The photophysical, electrochemical and photovoltaic properties of DSSC based on these dyes were systematically investigated and are correlated with their chemical structures.
|
Download:
|
| Scheme. 1.Molecular structures of truxene based dyes TX1,TX2 and TX3. | |
The 1H NMR and 13C NMR spectra were recorded on a BRUKER AVANCE III 600 MHz NMR instrument in CDCl3 and DMSO-d6, using tetramethylsilane as an internal reference. MALDI-TOF was performed on a Bruker Autoflex instrument,using 1,8,9-trihydroxyanthracene as a matrix. Elemental analyses of carbon, hydrogen,and nitrogen were performed on a Carlorerba-1106 microanalyzer. UV-vis absorption spectra were recorded on a spectrophotometer (UV-2450,Shimadzu). Electrochemical experiments were performed using a CH Instruments electrochemical workstation (model 660A). The potentials are quoted against the ferrocene internal standard. All starting materials were purchased from commercial suppliers (Sigma-Aldrich,J&K Scientific,and the Energy Chemical) and used without further purification. Tetrahydrofuran (THF) for synthesis was freshly distilled over Na-K alloy under argon prior to use. TiO2 paste and iodide-based liquid electrolyte (DHS-E23) was bought from Dalian Heptachroma SolarTech. Co.,Ltd.,China. 2.2. Synthesis
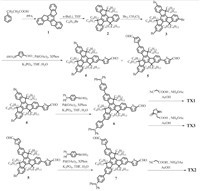
The general synthetic routes of the intermediates and the final compounds are outlined in Scheme 2. 10,15-Dihydro-5H-diindeno[ 1,2-a;1',2'-c]fluorene (truxene) (1) [28, 29],5,5',1',,10',15, 15'-hexahexyltruxene (2) [30] and 2,7,12-tribromo-5,5',10,,10', 15,150-hexahexyltruxene (3) [31] were synthesized as described in the literature. The Suzuki coupling reaction between 5-formyl-2- thiopheneboronic acidand compound 3 affordedcompound 4 and 5. The intermediates (6 and 7) were synthesized by palladiumcatalyzed Suzuki coupling reaction of 4-(diphenylamino)phenylboronic acid and the corresponding substrates. The final compounds were synthesized in a similar way to literature report [29]. The products were fully characterized by 1H and 13C NMR spectroscopy, MALDI-TOF spectrometry and the elemental analysis as well. Unfortunately,13C NMR spectra of TX2 and TX3 were not able to be obtained due to the poor solubility of these compounds.
|
Download:
|
| Scheme. 2.Synthetic routes for the three truxene-based dyes TX1-TX3. | |
A mixture of compound 3 (563 mg,0.519 mmol),5-formyl-2- thiophene-boronic acid (122 mg,0.778 mmol),Pd(OAc)2 (3.6 mg, 0.016 μmol),XPhos (9.1 mg,0.019 mmol) and aqueous solution of K3PO4 (2 mol L-1,4 mL) in THF (12 mL) were placed in a Schlenk tube under an argon atmosphere and was stirred at 60 °C for 12 h. After cooling,the reaction was quenched by adding water,and then was extracted with chloroform. The organic layer was washed with brine and dried over anhydrous Na2SO4. After evaporation of solvents,the residue was purified by column chromatography over silica gel using hexane/chloroform mixture (2:1) as the eluent to give the main product (compound 4,307 mg,53%,yellow solid) and the byproduct (compound 5,71 mg,12%,yellow solid).
A mixture of compound 3 (558 mg,0.515 mmol),5-formyl-2- thiophene-boronic acid (161 mg,1.03 mmol),Pd(OAc)2 (4.7 mg, 0.021 mmol),XPhos (11.9 mg,0.025 mmol) and aqueous solution of K3PO4 (2 mol L-1,4 mL) in tetrahydrofuran (12 mL) were placed in a Schlenk tube under an argon atmosphere and was stirred at 60 °C for 12 h. After cooling to room temperature,the reaction was quenched by adding water,and then was extracted with chloroform. The organic layer was washed with brine and dried over anhydrous Na2SO4. After the solvents were evaporated,the residue was purified by column chromatography over silica gel using hexane/chloroform mixture (2:1) as the eluent to give the main product (compound 5,254 mg,43%,yellow solid) and the byproduct (compound 4,98 mg,17%,yellow solid).
Compound 4: 1H NMR (600 MHz,CDCl3): δ 9.93 (s,1H,CHO), 8.38 (d,1H,J = 7.8 Hz),8.20 (dd,2H,J = 13.2 Hz,J = 9.0 Hz),7.80 (d, 2H,J = 3.6 Hz),7.75-7.73 (m,2H),7.58 (dd,2H,J = 5.4 Hz, J = 1.8 Hz),7.55-7.51 (m,2H),2.93-2.83 (m,6H),2.13-2.00 (m, 6H),0.93-0.82 (m,40H),0.63-0.49 (m,26H). 13C NMR (150 MHz, CDCl3): δ 181.40,154.71,153.60,153.53,144.55,144.17,141.18, 140.32,137.76,136.68,136.26,130.25,128.32,124.88,124.48, 124.03,123.71,122.85,120.01,118.80,55.00,54.82,35.78,30.31, 28.27,22.85,21.13,12.73. Anal. calcd. for C68H90Br2OS (%):C,73.23; H,8.13. Found: C,73.59; H,7.98. MALDITOF-MS: m/z calcd. 1115.3,found 1115.1 (M+). Compound 5: 1H NMR (600 MHz,CDCl3): δ 9.95 (s,2H,CHO), 8.39-8.33 (m,3H),7.82 (d,2H,J = 4.2 Hz),7.79-7.77 (m,4H),7.66 (dd,2H,J = 5.4 Hz,J = 1.8 Hz),7.58 (s,2H),3.04-2.92 (m,6H),2.24- 2.10 (m,6H),0.94-0.87 (m,48H),0.64-0.58 (m,18H). 13C NMR (150 MHz,CDCl3): δ 182.60,154.70,154.65,154.47,145.75, 145.34,143.72,142.71,141.42,138.66,136.47,132.09,128.58, 127.64,125.88,124.63,124.26,124.10,123.93,123.14,120.17, 119.66,55.87,37.02,36.84,31.47,29.44,24.04,22.30,13.89. Anal. calcd. for C73H93BrO2S2 (%): C,76.47; H,8.18. Found: C,76.62; H, 8.10. MALDITOF-MS: m/z calcd. 1146.6,found 1147.1 (M++1).
2.2.2. Synthesis of compound 6A mixture of compound 4 (463 mg,0.415 mmol),4-(diphenylamino) phenylboronic acid (300 mg,1.04 mmol),Pd(OAc)2 (4.0 mg,0.02 mmol),XPhos (9.5 mg,0.025 mmol) and aqueous solution of K3PO4 (2 mol L-1,3 mL) in THF (10 mL) were placed in a Schlenk tube under an argon atmosphere and was stirred at 60 °C for 12 h. After cooling,the reaction was quenched by adding water, and then was extracted with chloroform. The organic layer was washed with brine and dried over anhydrous Na2SO4. After solvents were evaporated,the residue was purified by column chromatography over silica gel using hexane/chloroform mixture (2:1) as the eluent to give the product (450 mg,75%) as a yellow solid. 1H NMR (600 MHz,CDCl3): δ 9.91 (s,1H,CHO),8.43-8.37 (m, 2H),7.78 (d,1H,J = 3.0 Hz),7.75-7.74 (m,2H),7.65-7.63 (m,8H), 7.54 (d,1H,J = 3.0 Hz),7.27 (t,9H,J = 7.8 Hz),7.20 (d,4H, J = 6.0 Hz),7.16 (d,8H,J = 6.0 Hz),7.03 (t,4H,J = 7.2 Hz),3.02-2.94 (m,6H),2.17-2.12 (m,6H),0.93-0.84 (m,36H),0.60-0.47 (m, 30H). 13C NMR (150 MHz,CDCl3): δ 181.42,153.84,153.62,153.11, 152.99,146.58,146.05,144.68,144.26,143.88,140.91,137.84, 137.77,137.53,137.34,137.12,136.28,136.21,134.03,129.78, 128.12,126.52,123.97,123.79,123.66,123.54,123.24,123.92, 122.67,121.78,118.89,118.83,118.70,55.10,36.10,35.91,30.31, 28.31,22.81,21.08,12.67. Anal. calcd. for C104H118N2OS (%): C, 86.50; H,8.24; N,1.94; Found: C,86.94; H,7.90; N,2.21. MALDITOF-MS: m/z calcd. 1444.1,found 1444.6 (M+).
2.2.3. Synthesis of compound 7A mixture of compound 5 (198 mg,0.174 mmol),4-(diphenylamino) phenylboronic acid (65 mg,0.225 mmol),Pd(OAc)2 (2.4 mg,0.01 mmol),XPhos (6.0 mg,0.013 mmol) and aqueous solution of K3PO4 (2 mol L-1,3 mL) in tetrahydrofuran (10 mL) were placed in a schlenk tube under a argon atmosphere and was stirred at 60 °C for 12 h. After cooling,the reaction was quenched by adding water,and then was extracted with chloroform. The organic layer was washed with brine and dried over anhydrous Na2SO4. After solvents were evaporated,the residue was purified by column chromatography over silica gel using hexane/chloroform mixture (1:1) as the eluent to give the product (205 mg,90%) as yellow solid. 1H NMR (600 MHz,CDCl3): δ 9.94 (s,2H,CHO), 8.44-8.38 (m,3H),7.80 (d,2H,J = 4.2 Hz),7.77-7.75 (m,4H),7.68- 7.64 (m,4H),7.56 (s,2H),7.31-7.28 (m,4H),7.22 (d,2H,J = 8.4 Hz), 7.18 (d,4H,J = 7.8 Hz),7.06 (t,2H,J = 3.9 Hz),3.03-2.91 (m,6H), 2.28-2.14 (m,6H),0.94-0.85 (m,48H),0.61-0.55 (m,18H). 13C NMR (150 MHz,CDCl3): δ 182.61,154.83,154.80,154.67,154.17, 147.75,147.34,146.31,145.90,145.46,142.25,141.78,141.71, 138.67,138.65,137.36,131.23,129.32,127.71,125.25,124.93, 124.47,124.06,123.91,123.02,120.08,119.97,55.97,37.14,36.97, 31.48,29.48,24.00,22.25,13.83. Anal. calcd. for C91H107NO2S2 (%): C,83.37; H,8.23; N,1.07; Found: C,83.73; H,8.05; N,1.34. MALDITOF-MS: m/z calcd. 1310.9,found 1311.5 (M+).
2.2.4. Synthesis of compound TX1A mixture of compound 6 (216 mg,0.150 mmol),cyanoacetic acid (38 mg,0.450 mmol),ammonium acetate (57 mg, 0.740 mmol),and acetic acid (20 mL) were heated at reflux for 6 h under argon atmosphere. After cooling to room temperature,it was precipitated by pouring into water. The resulting solid was filtered,washed thoroughly with water. Then,the crude product was purified by silica-gel column chromatography using chloroform/ ethanol mixture (10:1) as the eluent to afford TX1 as a yellow solid (188 mg,83%). 1H NMR (600 MHz,CDCl3): δ 8.48-8.39 (m, 3H),8.02 (dd,2H,J = 6.6 Hz,J = 3.0 Hz),7.87 (s,1H),7.81 (d,1H, J = 7.2 Hz),7.78 (s,1H),7.67 (s,2H),7.64 (d,6H,J = 7.8 Hz),7.62- 7.54 (m,3H),7.28 (t,8H,J = 7.2 Hz),7.20 (d,2H,J = 7.2 Hz),7.16 (d, 8H,J = 8.4 Hz),7.04 (t,3H,J = 7.2 Hz),3.03-2.95 (m,6H),2.20-2.17 (m,6H),9.94-0.85 (m,36H),0.60-0.48 (m,30H). 13C NMR (150 MHz,CDCl3): δ 154.86,154.12,147.66,147.12,145.90, 145.40,145.03,138.92,138.84,138.61,138.39,138.24,137.36, 135.11,129.22,127.61,124.33,124.01,122.86,121.50,55.92, 55.76,31.39,29.41,29.34,23.92,22.19,13.78. Anal. calcd. for C107H119N3O2S (%): C,85.04; H,7.94; N,2.78; Found: C,85.45; H, 7.49; N,2.99. MALDITOF-MS:m/z calcd. 1511.2,found 1511.1 (M+).
2.2.5. Synthesis of compound TX2A mixture of compound 7 (150 mg,0.114 mmol),cyanoacetic acid (109 mg,0.570 mmol),ammonium acetate (72 mg, 0.940 mmol),and acetic acid (20 mL) were heated at reflux for 10 h under argon atmosphere. After cooling to room temperature, it was precipitated by pouring into water. The resulting solid was filtered,washed thoroughly with water. Then,the crude product was purified by silica-gel column chromatography using chloroform/ ethanol mixture (10:1) as the eluent to afford TX1 as a deep yellow solid (109 mg,66%). 1H NMR (600 MHz,CDCl3): δ 8.44 (d,2H,J = 7.8 Hz),8.40 (d,2H,J = 7.8 Hz),8.37 (s,2H, CH = C(CN)(COOH)),7.83-7.80 (m,4H),7.77 (s,2H),7.68-7.65 (m,3H),7.58-7.56 (m,2H),7.31-7.28 (4H),7.22 (d,2H,J = 8.4 Hz), 7.18 (d,4H,J = 7.8 Hz),7.06 (t,2H,J = 7.2 Hz),3.03-2.90 (m,6H), 2.26-2.16 (m,6H),1.33-1.25 (m,20H),0.93-0.86 (m,28H),0.61- 0.54 (m,18H). Anal. calcd. for C97H109N3O4S2 (%): C,80.62; H,7.60; N,2.91; Found: C,80.90; H,7.88; N,2.74. MALDITOF-MS:m/z calcd. 1445.1,found 1445.1 (M+).
2.2.6. Synthesis of compound TX3A mixture of compound 6 (216 mg,0.150 mmol),rhodanine-3- acetic acid (86 mg,0.450 mmol),ammonium acetate (57 mg, 0.740 mmol),and acetic acid (20 mL) were heated at reflux for 6 h under argon atmosphere. After cooling to room temperature,it was precipitated by pouring into water. The resulting solid was filtered, washed thoroughly with water. Then,the crude product was purified by silica-gel column chromatography using chloroform/ ethanol mixture (15:1) as the eluent to afford TX3 as a red solid (182 mg,75%). 1H NMR (600 MHz,DMSO-d6): δ 8.40 (br,3H),8.21 (br,2H),8.12-8.10 (m,6H),8.06 (br,2H),7.97 (br,2H),7.91-7.89 (br,6H),7.78-7.74 (m,10H),7.34 (br,4H),7.08 (br,6H),4.75 (s,2H, CH2COOH),3.00 (br,6H),2.26 (br,6H),1.29 (br,4H),0.92-0.82 (br, 32H),0.51 (br,30H). Anal. calcd. for C109H121N3O3S3 (%): C,80.95; H,7.54; N,2.60; Found: 81.17; H,7.35; N,2.99. MALDITOF-MS:m/z calcd. 1615.9,found 1614.2 (M+-1).
2.3. Device fabrication and characterizationTo make a reasonable comparison,all the anode films for the DSSCs were made under the same standard manner,which are composed of a 12 μm thick transparent layer (TiO2 with diameter of 20 nm) and a 6 μm thick scattering layer (TiO2 nanoparticles with a diameter of 200 nm). Specifically,a doctor-blade technique was utilized to prepare photoanode (TiO2) films. First,a layer of ∼6 μm TiO2 paste (20 nm) was doctor-bladed onto the FTO conducting glass and then relaxed at room temperature for 3 min before heating at 150 °C for 6 min; this procedure was repeated once to achieve a film thickness of ∼12 μm and the resulting surface was finally coated by a scattering layer (∼6 μm) of TiO2 paste (200 nm). The electrodes were gradually heated under an air flow at a rate of ∼+55 °C/5 min and 480 °C for 45 min to remove polymers and generate a three dimensional TiO2 nanoparticle network. After that,the sintered films were soaked with 0.02 mol L-1 TiF4 aqueous solution for 45 min at 70 °C,washed with deionized water,and further annealed at 450 °C for 30 min.
After cooling down to∼80 °C,the electrodeswere immersed into a 0.3mmol L-1 dye bathinTHF/EtOH(volumeratio,1:1) solution for the truxene dye series and maintained in the dark for 18 h. Afterward,the electrodes were rinsed with ethanol to remove the non-adsorbed dyes and dried in the air. Pt counter electrodes were prepared by sputtering method at 13mA for 180 s at a power of 150W. Two holes (0.75mmin diameter)were predrilled in the FTO glass for introducing the electrolyte. The dye-adsorbed TiO2 electrode and Pt-counter electrodewere assembled into a sandwich type cell and sealed with a hot-melt parafilm at about 100 °C. The liquid electrolyte consisting of 0.6 mol L-1 1,2-dimethyl-3-propylimidazolium iodide (DMPII),0.1 mol L-1 LiI,0.05mol L-1 I2 in a mixture of acetonitrile and 4-tert-butylpyridine (volume ratio,1:1) was introduced into the cell through the drilled holes at the back of the counter electrode. At last,the holeswere sealed by parafilm and covering glass (0.1mm thickness) at elevated temperature. The effective areas of all the TiO2 electrodes were 0.196 cm2. The current-voltage (J-V) characteristics of the assembled DSSCs were measured by a semiconductor characterization system (Keithley 236) at roomtemperature in air under the spectral output fromsolar simulator (Newport) using an AM 1.5G filter with a light power of 100 mW/cm2. IPCEs ofDSSCswere recorded inthe Solar CellQE/IPCE Measurement System(Zolix Solar Cell Scan 100) using dc mode. CHI 760E electrochemical workstation was used to characterize the electrochemical properties of the DSSCs. Electrochemical impedance spectroscopy (EIS) was recorded under dark conditions over a frequency range of 0.1-105 Hz with an AC amplitude of 10 mV,and the parameters were calculated from Z-View software (v2.1b, Scribner AssociatNe,Inc.). For the open-circuit voltage decay measurements,the cell was first illuminated for 20 s to a steady voltage,then the illumination was turned off for 80 s and the opencircuit voltage decay curve was recorded.
3. Results and discussion 3.1. UV-vis absorption spectraThe UV-vis absorption of TX1,TX2 and TX3 in chloroform (CHCl3) is shown in Fig. 1a,and two absorption bands were observed. The one below 400 nm is corresponding to the φ-φ* transition of the whole conjugation system,and the one located at longer wavelengths (about 440 nm for TX1 and TX2,and 480 nm for TX3) is assigned to the intramolecular charge transfer (ICT) band. As shown in Table 1,the ICT absorption maxima (lmax) appear at 438,440 and 480 nm for TX1,TX2 and TX3, respectively. Apparently,the dye with rhodanine-3-acetic acid as the anchoring group shows the largest red-shifts of absorption bands in chloroform compared to the other two dyes with cyanoacetic acid,indicating that the rhodanine 3-acetic acid acceptor leads to a stronger ICT in TX3. Dye TX1 and TX2 have similar absorption spectra,suggesting that the amount of acceptor groups has little effect on the charge transfer process in this molecular system. The molar extinction coefficients (e) at lmax of the absorption spectra for TX1-3 are 48357,45209,and 27293 L mol-1 cm-1,respectively. Detailed photophysical values are presented in Table 1. Fig. 1b depicts the absorption spectra of the dyes adsorbed onto TiO2 films. The φ-φ* transition bands of the three dyes red shifted,while the ICT bands blue shifted. The red shift of the φ-φ* transition should be ascribed from the dye aggregation. The blue shifted ICT band should be due to the dye- TiO2 interaction. According to literature report,after the dyes are adsorbed on TiO2,the deprotonation of the carboxylate group due to the dye-TiO2 interaction will weaken the ability of the acceptor (carboxylate-TiO2) compared to the carboxylic acid, which could also cause the blue-shifted absorption peak [32]. It is worth noting that when dye TX3 is adsorbed onto TiO2 film, the charge transfer absorption band became significantly decreased,and this definitely will bring about negative effect toward the device performance. In strong contrast,the absorption spectrum of dye TX2 on TiO2 is broader in comparison to that in solution,and moreover,it is the strongest among the dyes studied,which is an advantageous spectral property for light harvesting of the solar irradiation.
| Table 1 Electrochemical and photophysical properties of TX1,TX2 and TX3 dyes. |

|
Download:
|
| Fig. 1.Absorption of TX1,TX2 and TX3 in chloroform (a) and on TiO2 film (b). | |
The electrochemical behaviors of the dyes were measured by cyclic voltammetry. The relevant CV data were presented in Table 1. Cyclic voltammetry in CH2Cl2 solution was used to determine the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbitals (LUMOs) of the dyes. The ground oxidation potentials (Eox) correspond to the highest occupied molecular orbitals (HOMO). The lowest unoccupied molecular orbitals (LUMO) were obtained from the values of Eox and the zero-zero band gaps (E0-0) estimated from the onset of the UV-visible absorption spectra. The HOMO and LUMO levels of the dyes are collected in Table 1. The HOMO levels of all dyes are more positive than iodide/tri-iodide redox potential value (0.4ωvs. NHE),indicating that the oxidized dyes could be efficiently regenerated by the electrolyte. Their LUMO levels are estimated from the HOMOs and the zero-zero band gaps (E0-0),and the values are higher than the conduction band (CB) of the TiO2 anode (at approximately -4.00 eV),suggesting efficient electron injection ability from the LUMO of the dyes to the CB band of TiO2 semiconductor. Therefore,these dyes can be used as sensitizers for feasible electron transfer in DSSCs.
y3.3. Molecular orbital calculationsThe structures of the three dyes have been analyzed by Gaussian 09 at B3LYP/6-31+G(d) level [33, 34] for full geometrical optimization (Fig. 2). Obviously,the HOMO levels are mainly delocalized on the triphenylamine groups,and also part on the truxene core,while the LUMO levels are mainly located on the acceptor and the neighboring spacers,as well as partly on the tuxene core. The orbital overlap between the donor and the acceptor is able to afford efficient charge transition. When these dyes are adsorbed onto the TiO2 surface,the photo-generated electrons can be injected into the conduction band of the semiconductor from the acceptor unit via the terminal cyanoacrylic acid or the rhodanine 3-acetic acid anchoring group. It is noteworthy that in TX3,the LUMO orbital in only partly distributed on the rhodanine-3-acetic acid (mainly on the heterocyclic part), which makes inefficient electron injection from the LUMO orbital to the conduction band (CB) of TiO2. As a result,the absence of orbital distribution on the anchoring group in TX3 may lead to poor solar cell performance.

|
Download:
|
| Fig. 2.Frontier molecular orbitals of the HOMO and LUMO of the three dyes calculated by DFT on a B3LYP/6-31+G(d)* level. | |
The typical current-voltage characteristics of DSSCs sensitized with these three dyes on TiO2 films with I-/I3- electrolyte measured under standard AM 1.5G conditions (100mWcm-2) are shown in Fig. 3a. Among the three sensitizers,dye TX2 exhibits the highest power conversion efficiency of 2.64%,with a short circuit current density (Jsc) of 5.09 mA cm-2,and the open circuit potential (Voc) of 729 mV. TX1 under the same condition shows h of 2.24%,with Jsc of 4.68 mA cm-2 and Voc of 685 mV. While the TX3 with the rhodanine 3-acetic acid anchoring group delivers the lowest h of 1.15%,with Jsc of 3.15 mA cm-2 and Voc of 580 mV. The photovoltaic parameters of these dyes are summarized in Table 2. The observed trend can be rationalized by the photo responses of the devices based on these dyes as studied by incident photon to current conversion efficiency (IPCE) spectra shown in Fig. 3b. The IPCE curves of DSSCs based on TX1-TX3 exhibit absorption in the range of 300-600 nm. The dyes show broader IPCE range which is consistent with the absorption spectra. Among the three dyes,TX2 exhibits the highest IPCE value,perhaps due to its better adsorption on the TiO2 film as a result of the two anchoring groups. And this also leads to higher Jsc value of TX2. Compared to TX1 and TX2,the rhodanine 3-acetic acid group in TX3 resulted in the lowest efficiency,probably due to the fact that the rhodanine- 3-acetic acid as an anchoring group in TX3 induces a diagonal orientation of the dye and produces mostly slanted dipole direction relative to the oxide electrode [yes]. The performance of the three dyes sensitized solar cells could be further verified by the EIS results that will be discussed in the following part.
| Table 2 Photovoltaic performance of DSSCs based on dyes TX1,TX2 and TX3. |

|
Download:
|
| Fig. 3.(a) Photocurrent density-photovoltage curves of TX1,TX2 and TX3 under AM 1.5G simulated sunlight (100mWcm-2) illumination and (b) IPCE spectra of TX1,TX2 and TX3 based devices. | |
It has been reported that the number of the anchored sensitizers on the TiO2 film critically influences the Jsc aswell,the dye loading of the three sensitizers was determined. The TiO2 film was soaked in 0.3mmol L-1 organic dyes in THF/ethanol (1:1 v/v) for 18 h in dark, and the UV-vis absorbance of the remaining solutionwas measured. The dye loading of each device is then estimated from the Beer- Lambert’s law with the help of the dye’s respective extinction coefficient. The dye loading of each device is estimated to be ∼1.93 x 10-7,∼1.85 x 10-7 and ∼1.14 x 10-7 mol cm-2 for TX1, TX2 and TX3,respectively.
Electrochemical impedance spectroscopy (EIS) is also performed to elucidate the interfacial charge recombination processes in DSSCs based on these dyes under the dark conditions. Fig. 4 shows the Nyquist and the OCVD (open-circuit voltage decay) profiles for DSSCs based on TX1,TX2 and TX3 measured under the dark condition. The larger semicircle in the lower frequency range represents the resistance of the recombination between electrons on the TiO2 conduction band and I3- species in the electrolyte at the TiO2/dye/electrolyte interface [yes]. The calculated resistance values (Rrec) are 155.94ωfor TX1,347.35ωfor TX2 and 26.80 V for TX3. Generally,the larger the Rrec,the slower the recombination kinetics. Therefore,charge recombination in TX2 could be more efficiently blocked among the three dyes. Apart from the charge transfer resistance mentioned above,the electron lifetime (t) is another important parameter for DSSCs,which could be extracted from the slope of Voc decay curves [yes]. As a consequence,for devices sensitized with TX1,TX2 and TX3,the slopes become steeper in TX3 and TX1 than that in TX2,indicating that the electron lifetimes are gradually reduced in the order of TX2 > TX1 > TX3,which is consistent with the EIS spectra,as well as their open circuit voltage results.

|
Download:
|
| Fig. 4.EIS Nyquist plots for DSSCs based on the three dyes under dark condition (a) and open-circuit voltage decay profiles of DSSCs based on the dyes. | |
In conclusion,three new organic dyes based on truxene core structure were synthesized containing triphenylamine as the electron donor,thiophene rings as the φ spacer,and the cyanoacetic acid or rhodanine 3-acetic acid as the electron acceptor. Among the three dyes,dye TX2 having two cyanoacetic acid acceptors outperforms the other two dyes. It has broader and stronger absorption on TiO2 film. The EIS results also indicate a slower charge recombination processes,and Voc decay curves indicate a longer electron lifetime in TX2. As a result,it exhibits the best efficiency of 2.64%,with Jsc = 5.09 mA cm-2,Voc = 729 mV, FF = 71.1. Whereas,dye TX3 with rhodanine-3-acetic acid as the anchoring group delivers the lowest power conversion efficiency, due to its poor absorption on TiO2 film and its shorter electron lifetime,as well as the fast charge recombination from the EIS results. We believe that the truxene-based dyes could be further modified to achieve higher power conversion efficiency.
AcknowledgmentsThis work is supported by ‘‘Fundamental Research Funds for the Central Universities’’ (Nos. XDJK2014C145 and XDJK2014C052), and the Starting Foundation of Southwest University (Nos. SWU113076 and SWU113078). L. Zhu thanks the financial support from National Natural Science Foundation of China (No. 51203046).
| [1] | B. O'Regan, M. Grä tzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature 353 (1991) 737-740. |
| [2] | M. Grä tzel, Recent advances in sensitized mesoscopic solar cells, Acc. Chem. Res. 42 (2009) 1788-1798. |
| [3] | A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, H. Pettersson, Dye-sensitized solar cells, Chem. Rev. 110 (2010) 6595-6663. |
| [4] | C.Y. Chen, M.K. Wang, J.Y. Li, et al., Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells, ACS Nano 3 (2009) 3103-3109. |
| [5] | M.K. Nazeeruddin, F. De Angelis, S. Fantacci, et al., Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers, J. Am. Chem. Soc. 127 (2005) 16835-16847. |
| [6] | A. Yella, H.W. Lee, H.N. Tsao, et al., Porphyrin-sensitized solar cells with cobalt (II/ III)-based redox electrolyte exceed 12 percent efficiency, Science 334 (2011) 629-634. |
| [7] | H. Li, T.M. Koh, A. Hagfeldt, et al., New donor-π-acceptor sensitizers containing 5H-[1,2,5]thiadiazolo [3,4-f]isoindole-5, 7(6H)-dione and 6H-pyrrolo[3,4-g]quinoxaline-6, 8(7H)-dione units, Chem. Commun. 49 (2013) 2409-2411. |
| [8] | Y. Bai, J. Zhang, D. Zhou, et al., Engineering organic sensitizers for iodine-free dyesensitized solar cells: red-shifted current response concomitant with attenuated charge recombination, J. Am. Chem. Soc. 133 (2011) 11442-11445. |
| [9] | W. Hung, Y.Y. Liao, T.H. Lee, et al., Eugenic metal-free sensitizers with double anchors for high performance dye-sensitized solar cells, Chem. Commun. 51 (2015) 2152-2155. |
| [10] | Y.R. Gao, L.L. Chu, W. Guo, T.L. Ma, Synthesis and photoelectric properties of an organic dye containing benzo[1,2-b: 4, 5-b']dithiophene for dye-sensitized solar cells, Chin. Chem. Lett. 24 (2013) 149-152. |
| [11] | Y. Hua, S. Chang, D.D. Huang, et al., Significant improvement of dye-sensitized solar cell performance using simple phenothiazine-based dyes, Chem. Mater. 25 (2013) 2146-2153. |
| [12] | M.W. Lee, J.Y. Kim, D.H. Lee, M.J. Ko, Novel D-π-A organic dyes with thieno[3,2-b]thiophene-3, 4-ethylenedioxythiophene unit as a π-bridge for highly efficient dye-sensitized solar cells with long-term stability, ACS Appl. Mater. Inter. 6 (2014) 4102-4108. |
| [13] | Y.Y. Gao, C.J. You, J.P. Chen, et al., Synthesis of a polymer-bound sensitizer and its application in the photoisomerization of trans-vitamin D3 to cis-vitamin D3, Chin. Chem. Lett. 13 (2002) 1158-1161. |
| [14] | Z.J. Ning, Q. Zhang, H.C. Pei, et al., Photovoltage improvement for dye-sensitized solar cells via cone-shaped structural design, J. Phys. Chem. C 113 (2009) 10307-10313. |
| [15] | J.L. Wang, Z.M. Tang, Q. Xiao, Y. Ma, J. Pei, Star-shaped D-π-A conjugated molecules: synthesis and broad absorption bands, Org. Lett. 11 (2009) 863-866. |
| [16] | X.Y. Cao, X.H. Zhou, H. Zi, J. Pei, Novel blue-light-emitting truxene-containing hyperbranched and zigzag type copolymers: synthesis, optical properties, and investigation of thermal spectral stability, Macromolecules 37 (2004) 8874-8882. |
| [17] | Z.F. Yang, B. Xu, J.T. He, et al., Solution-processable and thermal-stable triphenylamine-based dendrimers with truxene cores as hole-transporting materials for organic light-emitting devices, Org. Elect. 10 (2009) 954-959. |
| [18] | W.Y. Lai, R.D. Xia, Q.Y. He, et al., Enhanced solid-state luminescence and lowthreshold lasing from starburst macromolecular materials, Adv. Mater. 21 (2009) 355-360. |
| [19] | Y.M. Sun, K. Xiao, Y.Q. Liu, et al., Oligothiophene-functionalized truxene: starshaped compounds for organic field-effect transistors, Adv. Funct. Mater. 15 (2005) 818-822. |
| [20] | X.Y. Yang, Q.D. Zheng, C.Q. Tang, et al., Star-shaped chromophores based on a benzodithiophene fused truxene core for solution processed organic solar cells, Dyes Pigments 99 (2013) 366-373. |
| [21] | Y.J. Hao, M. Liang, Z.H. Wang, et al., Synthesis of new truxene based organic sensitizers for iodine-free dye-sensitized solar cells, Tetrahedron 69 (2013) 10573-10580. |
| [22] | X.P. Zong, M. Liang, T. Chen, et al., Efficient iodine-free dye-sensitized solar cells employing truxene-based organic dyes, Chem. Commun. 48 (2012) 6645-6647. |
| [23] | C.B. Nielsen, E. Voroshazi, S. Holliday, et al., Electron-deficient truxenone derivatives and their use in organic photovoltaics, J. Mater. Chem. A 2 (2014) 12348-12354. |
| [24] | S.H. Lin, Y.C. Hsu, J.T. Lin, C.K. Lin, J.S. Yang, Isotruxene-derived cone-shaped organic dyes for dye-sensitized solar cells, J. Org. Chem. 75 (2010) 7877-7886. |
| [25] | L.N. Zhu, H.B. Yang, C. Zhong, C.M. Li, Modified triphenylamine-dicyanovinylbased donor-acceptor dyes with enhanced power conversion efficiency of p-type dye-sensitized solar cells, Chem. Asian J. 7 (2012) 2791-2795. |
| [26] | M.S. Yuan, Q. Fang, Z.Q. Liu, et al., Acceptor or donor (diaryl B or N) substituted octupolar truxene: synthesis, structure, and charge-transfer-enhanced fluorescence, J. Org. Chem. 71 (2006) 7858-7861. |
| [27] | A.L. Kanibolotsky, R. Berridge, P.J. Skabara, et al., Synthesis and properties of monodisperse oligofluorene-functionalized truxenes: highly fluorescent starshaped architectures, J. Am. Chem. Soc. 126 (2004) 13695-13702. |
| [28] | W.H. Liu, I.C. Wu, C.H. Lai, et al., Simple organic molecules bearing a 3,4-ethylenedioxythiophene linker for efficient dye-sensitized solar cells, Chem. Commun. (2008) 5152-5154. |
| [29] | J.K. Zhao, Y.G. Wang, A facile and efficient synthesis of N,N-dimethylarylamines from aryl bromides, Chin. Chem. Lett. 13 (2002) 1149-1151. |
| [30] | Z.Q. Wan, L.L. Zhou, C.Y. Jia, et al., Comparative study on photovoltaic properties of imidazole-based dyes containing varying electron acceptors in dye-sensitized solar cells, Synth. Met. 196 (2014) 193-198. |
| [31] | M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford, 2009. |
| [32] | J.J. He, G. Benkö , F. Korodi, et al., Modified phthalocyanines for efficient near-IR sensitization of nanostructured TiO2 electrode, J. Am. Chem. Soc. 124 (2002) 4922-4932. |
| [33] | X. Qian, Y.Z. Zhu, J. Song, X.P. Gao, J.Y. Zheng, New donor-π-acceptor type triazatruxene derivatives for highly efficient dye-sensitized solar cells, Org. Lett. 15 (2013) 6034-6037. |
| [34] | J. van de Lagemaat, N.G. Park, A.J. Frank, Influence of electrical potential distribution, charge transport, and recombination on the photopotential and photocurrent conversion efficiency of dye-sensitized nanocrystalline TiO2 solar cells: a study by electrical impedance and optical modulation techniques, J. Phys. Chem. B 104 (2000) 2044-2052. |