b College of Pharmacy, Shandong University of Traditional Chinese Medicine, Jinan 250355, China;
c Department of Medicinal Chemistry, Beijing Key Laboratory of Active Substances Discovery and Drugability Evaluation, Institute of Materia Medica, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing 100050, China
Tuberculosis (TB) is a chronic infectious disease that seriously threatens human health. Moreover,in the past decade worldwide efforts have been made to treat TB due to the fast increasing population of TB,the emergence of drug-resistant TB,and the worldwide HIV and TB co-infection [1, 2]. In 2012,it is estimated that 8.6 million people developed the disease of TB,among them 13% were also proved to be HIV-positive. The situation becomes more serious since an estimated of 450,000 people in 2012 developed multi-drug resistant TB (MDR-TB) and an estimated 170,000 died of MDR-TB [3].
Currently,regimens for the treatment of TB which the bacteria are antibiotic susceptible must contain multiple first-line antituberculosis drugs such as isoniazid,rifampicin,pyrazinamide and ethambutol [3]. Whereas,the treatment of multi-drug resistant TB is more complicated,consists of what are called second-line drugs which are more expensive than first-line drugs and have more adverse effects [4]. Generally,the treatment procedure can take up to two years,and one third of MDR-TB patients will unfortunately eventually die of this disease [5]. Therefore,it is urgent to develop novel anti-tuberculosis agents with high efficacy and low toxicity, particularly with different mode of actions compared to current existed anti-TB drugs.
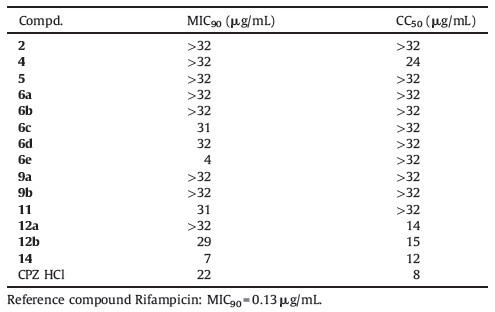
Phenothiazines are used in clinic as an effective antipsychotic for the treatment of psychosis for about 60 years. Interestingly,phenothiazines were also reported of in vitro activities of anti-tumor [6],anti-bacterial [7],anti-plasmid [8] and anti-tuberculosis [9, 10]. Previous reports have demonstrated in vitro and in vivo activity of some known phenothiazine derivatives,such as promethazine,chlorpromazine (CPZ),trifluoperazine and thioridazine (TZ) (Fig. 1) against drug-susceptible and drug-resistant TB bacteria [11, 12]. There are also confirmed reports of a TB patient cured with CPZ [13]. However, significant side effects especially extrapyramidal motor symptoms (EPMS) which comprises hyperkinetic-dystonic syndrome, Parkinson syndrome and Tardive dyskinesia constrained the application of phenothiazine derivatives in clinic [14]. Unfortunately, these psychotic related side effects is likely associated with or even a necessary condition for anti-psychotic efficacy [14, 15]. Moreover,the anti-tuberculosis MIC90 of CPZ analogs ranges from 0.9 mg/L to 32 mg/L,which exceeds the maximum safe serum level (0.5 mg/L) acceptable for the patient with psychotic [9]. Thus,it is necessary to eliminate the anti-psychotic efficacy and EPMS in order to develop this series of compounds as new anti-tuberculosis drugs.
|
Download:
|
| Fig. 1.10H-phenothiazine and phenothiazines. | |
The anti-tuberculosis activity of phenothiazine skeleton has not yet been properly explored. However,comparison of SARs of antituberculosis and anti-psychotic obtained from limited phenothiazine derivatives suggests that anti-TB activity is mainly from the scaffold of phenothiazine,since there is no obvious SAR trend can be concluded from the side chain and substituents of related phenothiazine derivatives [16, 17]. It is revealed that the terminal amine group in the 10-side chain as well as C-2 substitution determines the optimal neuroleptic activity on central nervous system [18].
With the clues of marginal anti-tuberculosis activity of phenothiazine compounds,we proposed to discover new anti- TB drugs by investigation of the relationship between the structure of phenothiazine derivatives and their anti-tuberculosis activities and further designed new compounds based on the information.
2. Experimental 2.1. Inhibitor design
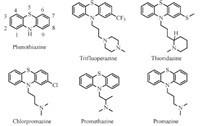
It is known that the side chain with an amino group is necessary for anti-psychotic activity of phenothiazine derivatives [18, 19],but it may not be necessary for anti-tuberculosis activity. We first explored the possibility to change the side chain,including removing the amino group. Then,further modification on the tricyclic ring and the side chain will be carried out. For series 1,we replaced 10-substituents with different non-basic substituents to eliminate the corresponding side effects. In addition,based on structure transformation of CPZ,we also did slightly modification on the phenothiazine core to decrease the conjugation system or replace phenothiazine with thioxanthenes and 9H-thioxanthene (Fig. 2).
|
Download:
|
| Fig. 2.The design strategy of series 1. | |
For series 2,we are aware of structure similarity of the basic moiety of 10-substituted side chain between CPZ and TM207. TMC207 was approved by the FDA with a MIC90 of 0.06 μg/mL in 2012 [20]. In mouse models of TB infection,TMC207 exceeded the anti-tuberculosis activities of WHO recommended combination of rifampin,isoniazid and pyrazinamide [21]. Given the highly potency of TMC207 as anti-tuberculosis agents,we extracted part of the moiety from TMC207 to replace the 10-substituted side chain of CPZ and designed 3-(dimethylamino)-1-(3-fluorophenyl)- 1-(9H-thioxanthen-9-yl) propan-1-ol as depicted (series 2) in Fig. 3. 2.2. Chemistry
The 1H NMR,13C NMR,HMBC,HMQC,H-HCOSY,DEPT were recorded on Mercury-300 and Mercury-400 spectrometer. Chemical shifts are reported as δ values with tetramethylsilane (TMS), employed as the internal standard. HR-ESIMS data were measured on Micromas AutoSpec Ultima-TOF spectrometer. 2.2.1. Series 1
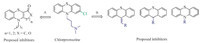
N-Alkyl derivatives 2,4,5 in series 1 were synthesized using phenothiazine as the starting material (Scheme 1). Compounds 2 and 4 was obtained through reported procedure [22]. Methylation of compound 4 with MeI/NaH gave 10-(3-methoxypropyl)-10Hphenothiazine 5 in the yield of 79.6%. The synthesis of 6a-d was achieved via the combination of phenothiazine with various reagents. Treatment of phenothiazine with NaH and 1-bromobutane or (3-bromopropoxy) benzene provided 6a and 6b in the yields of 56.4% and 63.1% respectively. Treatment of phenothiazine with 3-(1,3-dioxoisoindolin-2-yl)propanoyl chloride or benzoyl chloride in pyridine at 50 °C afforded 6c and 6d in the yields of 95.0% and 81.0%. Compound 6d was prepared through the treatment of phenothiazine with iodobenzene via coupling reaction in 87.3% yield.
|
Download:
|
| Scheme. 1.Synthesis of compounds 2-5 and 6a-6d. Reagents and conditions: (a) NaH,THF,r.t. 1 h,80.4%; (b) KOH,glycol/H2O,reflux 16 h,82.0%; (c) H2SO4,EtOH,reflux 6 h, 99.4%; (d) LiAlH4,THF,5 h,88.4%; (e) MeI,NaH,THF,2 h,79.6%; (f) for 6a (56.4%) and 6b (63.1%): NaH,r.t. 2 h; for 6c (95.0%) and 6d (81.0%): pyridine,50 °C,5 h; for 6e: Pd2(dba)3,DPPF,NaOBu,toluene,90 °C,16 h,87.3%. | |
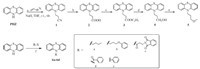
The design of compounds 9a and 9b are aimed to slightly modify the phenothiazine skeleton. Treatment 2(3H)-benzothiazolone with 1-bromopentane in NaOEt/EtOH under reflux afforded the intermediate 7 in 86.5% yield. Followed by hydrolysis in KOH/ EtOH under reflux for 2 h,intermediate 8 was then prepared in 98.0% yield. The synthesis of compound 9a and 9b was accomplished by condensation of 8 and cyclohexane-1,3-dione or tetronic acid in 76.7% and 93.1% yields respectively. 9Hthioxanthene analogs 11,12a-12b were synthesized as described in Scheme 2. The reduction of 9H-Thioxanthen-9-one with sodium borohydride in EtOH gave the intermediate of 10 in 94.4% yield. Followed by nucleophilic substitution with 1-(p-tolyl) urea, compound 11 was afforded in 95.9% yield. Compounds 12a and 12b were synthesized from 9H-thioxanthen-9-one with 3-hydroxy- 4-methoxybenzaldehyde via McMurry reaction in the yields of 38.2% and 63.9%,respectively.

|
Download:
|
| Scheme. 2.Synthesis of compounds 9a,9b,11 and 12a,12b. Reagents and conditions: (a) NaOEt,1-bromopentane,EtOH,reflux 4 h,86.5%; (b) KOH,EtOH,reflux 2 h,98.0%; (c) DMSO,155 °C,4 h; (d) sodium borohydride,EtOH,reflux 3 h,94.4%; (e) acetic acid,r.t. 2 h,95.9%; (f) Zn,TiCl4,aldehyde (R-CHO),THF,reflux,12a (38.2%),12b (63.9%). | |
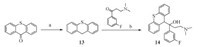
Series 2 was synthesized following Scheme 3. Treatment of 9Hthioxanthen- 9-one with borane tetrahydrofuran complex afforded intermediate 13 in 93.5% yield. Activation of 13 with n-BuLi and then reacted with 3-(dimethylamino)-1-(3-fluorophenyl) propan- 1-one to give compounds 14 in 40.2% yield.
|
Download:
|
| Scheme. 3.Synthesis of compounds 13,14. Reagents and conditions: (a) Borane tetrahydrofuran complex,THF,0 °C to r.t.,5 h,93.5%; (d) n-BuLi,-40 °C to r.t.,3 h, 40.2%. | |
All of these synthesized compounds were screened for anti-TB activity. The protocol of the anti-TB activity against M. tuberculosis H37Rv strain with the Microplate Alamar Blue Assay (MABA) was described in previous research [23]. The cytotoxicity against HepG2 cell lines were carried out following the method of literature [24]. All of the compounds were evaluated with the maximum concentration of 32 μg/mL. The results of anti-TB studies and cell cytotoxicity were listed in Table 1.
| Table 1 Anti-tuberculosis activity and cytotoxicity evaluations of synthesized compounds. |
Compound 6e showed the most effective anti-tuberculosis activity with MIC90 value of 4 μg/ml against M. tuberculosis H37Rv strain,which is very impressive to play as a lead compound for further development. Moreover,compound 6e did not show an obvious cytotoxicity at the concentration of 32 μg/ml,which enhances the potential to be further developed. In comparison, other compounds in series 1 with terminal alkyl (6a),hydroxyl (4), carboxyl (2),alkoxy (5) and phenoxy (6b) via an alkyl bridge show no anti-tuberculosis activity. And there were also no obvious improvement on the anti-tuberculosis activity when slightly modification on the phenothiazine core (9a,9b,11,12a) to discriminate the conjugation system or replace phenothiazine with thioxanthenes and 9H-thioxanthene. In addition,the weak anti-tuberculosis activity of compound 11 and 12b also implicate that a non-basic aryl substituents at the 10-position of phenothiazine ring can also maintain anti-tuberculosis activity. In summary, compared to the mother drug CPZ,phenothiazine derived compounds without 2-substituent and with 10-non-basic substituents can still remain or further increase the anti-TB activity,such as 6e. Importantly,these modifications are likely to eliminate the corresponding side effects such as anti-psychotic activity and EPMS.
In series 2,the representative compound 14 which adopts part of the moiety from TMC207 exhibits interesting anti-TB activity (7 μg/mL) as expected. However,compound 14 also show an obvious cyto-toxicity (Table 1). Thus,further development of this series of compounds was suspended. 4. Conclusion
Phenothiazine is known for its anti-psychotic pharmacological efficacy. Interestingly,these phenothiazine derivatives also show reasonable anti-TB activity but relative weak and with various psychotic related side effect. In this study,we designed two series of compounds,which derived from phenothiazine. And we found that 2-substituents and N,N-dimethyl amino terminal of phenothiazine drugs were not the essential elements to maintain the anti-TB activity,although they determined the optimal neuroleptic activity of the drugs. Our optimization also produced lead compound 6e with increased anti-TB activity (MIC90 = 4 mg/ mL,CC50 > 32 μg/ml) compared to mother compound CPZ (MIC90 = 22 μg/ml,CC50 = 8 μg/ml),but with much less toxic to mammalian cells. Compound 6e has a new structure moiety different from current known anti-TB drugs and can be used as the lead compound for further development of anti-TB agents.
Acknowledgment
We are grateful to ‘‘The National High-Tech Research and Development Program (‘‘863’’Program) of China’’ (No. 2012AA020302) for financial support. Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03. 027.
| [1] | A. Koul, E. Arnoult, N. Lounis, J. Guillemont, K. Andries, The challenge of new drug discovery for tuberculosis, Nature 469 (2011) 483-490. |
| [2] | M.D. Iseman, Tuberculosis therapy: past, present and future, Eur. Respir. J. Suppl. 36 (2002) 87s-94s. |
| [3] | WHO Publishes Global Tuberculosis Report 2013, Euro surveillance: bulletin Europeen sur les maladies transmissibles, Eur. Commun. Dis. Bull. 18 (2013). |
| [4] | C. Nathan, Drug-resistant tuberculosis: a new shot on goal, Nat. Med. 20 (2014) 121-123. |
| [5] | E. Pontali, A. Matteelli, G.B. Migliori, Drug-resistant tuberculosis, Curr. Opin. Pulm. Med. 19 (2013) 266-272. |
| [6] | N. Motohashi, S.R. Gollapudi, J. Emrani, K.R. Bhattiprolu, Antitumor properties of phenothiazines, Cancer Invest. 9 (1991) 305-319. |
| [7] | L. Amaral, M. Viveiros, J. Molnar, Antimicrobial activity of phenothiazines, In Vivo 18 (2004) 725-731. |
| [8] | C. Miskolci, I. Labadi, T. Kurihara, N. Motohashi, J. Molnar, Guanine-cytosine rich regions of plasmid DNA can be the target in anti-plasmid effect of phenothiazines, Int. J. Antimicrob. Agents 14 (2000) 243-247. |
| [9] | L. Amaral, J.E. Kristiansen, M. Viveiros, J. Atouguia, Activity of phenothiazines against antibiotic-resistant Mycobacterium tuberculosis: a review supporting further studies that may elucidate the potential use of thioridazine as anti-tuberculosis therapy, J. Antimicrob. Chemother. 47 (2001) 505-511. |
| [10] | P.B. Madrid, W.E. Polgar, L. Toll, M.J. Tanga, Synthesis and antitubercular activity of phenothiazines with reduced binding to dopamine and serotonin receptors, Bioorg. Med. Chem. Lett. 17 (2007) 3014-3017. |
| [11] | L. Amaral, A. Martins, G. Spengler, A. Hunyadi, J. Molnar, The mechanism by which the phenothiazine thioridazine contributes to cure problematic drug-resistant forms of pulmonary tuberculosis: recent patents for "new use", Recent Pat Antiinfective Drug Discov 8 (2013) 206-212. |
| [12] | L. Amaral, M. Martins, M. Viveiros, J. Molnar, J.E. Kristiansen, Promising therapy of XDR-TB/MDR-TB with thioridazine an inhibitor of bacterial efflux pumps, Curr. Drug Targets 9 (2008) 816-819. |
| [13] | L.E. Hollister, D.T. Eikenberry, S. Raffel, Chlorpromazine in nonpsychotic patients with pulmonary tuberculosis, Am. Rev. Respir. Dis. 81 (1960) 562-566. |
| [14] | B. Beckmann, H. Hippius, E. Ruther, Treatment of schizophrenia, Prog. Neuropsychopharmacol. 3 (1979) 47-52. |
| [15] | S. Kapur, R.B. Zipursky, G. Remington, Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia, Am. J. Psychiatry 156 (1999) 286-293. |
| [16] | P. Ratnakar, S.P. Rao, P. Sriramarao, P.S. Murthy, Structure-antitubercular activity relationship of phenothiazine-type calmodulin antagonists, Int. Clin. Psychopharmacol. 10 (1995) 39-43. |
| [17] | D. Addla, A. Jallapally, D. Gurram, et al., Rational design, synthesis and antitubercular evaluation of novel 2-(trifluoromethyl)phenothiazine-[1,2,3]triazole hybrids, Bioorg. Med. Chem. Lett. 24 (2014) 233-236. |
| [18] | A.P. Feinberg, S.H. Snyder, Phenothiazine drugs: structure-activity relationships explained by a conformation that mimics dopamine, Proc. Natl. Acad. Sci. U. S. A. 72 (1975) 1899-1903. |
| [19] | A. Jaszczyszyn, K. Gasiorowski, P. Swiatek, et al., Chemical structure of phenothiazines and their biological activity, Pharmacol. Rep. 64 (2012) 16-23. |
| [20] | E. Cox, K. Laessig, FDA approval of bedaquiline -the benefit-risk balance for drugresistant tuberculosis, N. Engl. J. Med. 371 (2014) 689-691. |
| [21] | K. Andries, P. Verhasselt, J. Guillemont, et al., A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis, Science 307 (2005) 223-227. |
| [22] | D. Cummins, G. Boschloo, M. Ryan, et al., Ultrafast electrochromic windows based on redox-chromophore modified nanostructured semiconducting and conducting films, J. Phys. Chem. B 104 (2000) 11449-11459. |
| [23] | Y. Lu, M. Zheng, B. Wang, et al., Clofazimine analogs with efficacy against experimental tuberculosis and reduced potential for accumulation, Antimicrob. Agents Chemother. 55 (2011) 5185-5193. |
| [24] | H. Cui, J. Carrero-Lerida, A.P. Silva, et al., Synthesis and evaluation of alphathymidine analogues as novel antimalarials, J. Med. Chem. 55 (2012) 10948-10957. |