b Medical College of Nanchang University, Nanchang 330006, China
Branched peptide is often used to mimic the conformational features of functional protein domains involved in biological molecular recognition,such as antigen-antibody,ligand-receptor, substrate-enzyme,adhesion protein pairs,and other protein- protein interaction. For instance,branched peptide with V3 loop domain of HIV surface glycoprotein gp120 was synthesized as a mimotope to display the V3 epitope for vaccine development [1], and demonstrated anti-HIV activity as well [2, 3]. A cyclic branched peptide derived from IgG Fc domain was reported its binding activity to the Fc gamma receptor I,while the linear peptides were inactive [4]. Another example is a branched cyclic peptide which indicated serine protease activity [5]. These and many other studies implicated that it is a good strategy using branched peptides as functional protein mimics.
Various synthetic approaches have been reported for preparation of branched peptides. The original strategy is to employ the selective protection and deprotection on the side-chain acid or amine of branch-site amino acids,then coupled with N-terminus amine or C-terminus acid of another protected peptide. NMethyltrityl is an acid-labile protecting group for Lys side-chain amino group and selective deprotection exposed the amine for introducing a new peptide on the branch [6]. Allyl group was used in protection of Glu and Asp side-chain acid and was selectively deprotected with palladium reagents [7, 8]. Using allyl-protected Glu and Dde-protected Lys,a branched cyclic peptide was successfully synthesized [9]. Selective protection approach requires the full protection of all other amino acids that limits its application especially for long peptides. Synthesis of branched peptide under unprotected condition has been well investigated. A conventional approach is to incorporate a functionalized unnatural amino acid during peptide synthesis and the function group enables site-specific conjugation with another functionalized peptide. Several bioorthogonal reactions were applied in branched peptide synthesis,such as click chemistry with peptide azide and alkyne [10, 11],native chemical ligation of peptide thioester and 4- mercapto-lysine [12],peptide hydrazide and aldehyde [13],etc.
Native chemical ligation of peptide thioester and N-terminus Cys peptide is wildly employed in long peptide or protein synthesis because of its efficient ligation of two unprotected peptides [14- 16]. However,based on our knowledge,ligation of Asp side-chain thioester and Cys peptide is not yet reported for branched peptide synthesis. This maybe due to the poor stability of thioester,that restrains the convenient preparation of peptide with side-chain thioester in solid-phase peptide synthesis (SPPS). Moreover, cyclization side-reaction of Asp side-chain acid to the nitrogen of neighbor amino acid [17, 18] complicated the site-chain ligation method.
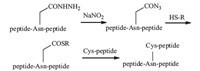
To develop a branched peptide synthetic approach via sitechain ligation,we sought to replace the thioester with a more stable precursor during SPPS. Recently,Liu and his co-workers published an elegant ligationmethod using peptide hydrazide as the precursor which was almost quantitatively converted to carbonyl azide and thioester after successive treatment with NaNO2 and MPAA [yes]. This method inspired us to develop a side-chain ligation approach for branched peptide synthesis using Asn sidechain hydrazide. As shown in Scheme 1,peptide bearing an Asnwith side-chain hydrazide was treated with NaNO2 and thiol reagent to form the side-chain thioester. The thioester in situ was ligated with an N-terminus Cys peptide giving a branched peptide. There are evident advantages of this new approach: Stable precursor of hydrazide in SPPS,efficient conversion from hydrazide to thioester, direct side-chain native chemical ligation with unprotected peptides,and expeditious assembly of branched loop peptide.
|
Download:
|
| Scheme. 1.Synthesis of branched peptide by Asn side-chain hydrazide ligation. | |
2. Experimental
Chemical reagents and solventswere purchased fromSinopharm Chemical Reagent Co. (Shanghai,China) and used without further purification. HPLC grade acetonitrile was purchased from Meryer (Shanghai,China). Fmoc-protected amino acids and peptide synthesis reagents were purchased from GL Biochem (Shanghai, China). Nuclear magnetic resonance (NMR) spectra were measured on a Varian-MERCURY Plus-400 instrument. The chemical shifts were assigned in ppm and the coupling constants in Hz. ESI-MS spectra were measured on an Agilent 6110 single quadrupole spectrometer. ESI-HRMS spectra were measured on an Agilent 6230 LC-TOF MS spectrometer. Analytical RP-HPLC was performed on a Beijing ChuangXinTongHeng LC3000 (analytic) instrument with a C18 column (5 μm,4.6mm x 150mm) at 40 °C. The column was elutedwith a linear gradient of [1TD$DIF]2%-90% acetonitrile in 30 min at a flow rate of 1mL/min. Preparative HPLC was performed on a Beijing Chuangxintongheng LC3000 (preparative) instrumentwith a preparative C18 column (10 μm,20mm x 300 mm). The column was eluted with a suitable gradient of aqueous acetonitrile containing 0.1% TFA at a flow rate of 10 mL/min. Solid-phase peptide synthesis was performed on a CS Bio CS336X peptide synthesizer. N-terminus Cys peptide 10 and12 were synthesized by Fmoc-chemistry following the standard procedure.
Synthesis of Fmoc-Asn(NHNHCbz)-OH (4) building block: Fmoc- Asp-OtBu (1,206 mg,0.5mmol) was pre-activated with HATU (190 mg,0.5mmol) and DIPEA (166 μL,1mmol) in DMF (3mL) for 30 min,then was added into a solution of benzyl hydrazinecarboxylate (2,83mg,0.5mmol) inDMF (2mL). The mixturewas stirred at roomtemperature for 3 h,and water (5mL) was added. The residue was extracted with EtOAc (3 x 5mL) and the organic layers [2TD$DIF]were combined and washed with water (5 mL) and brine (5mL),dried over anhydrous sodium sulfate. After filtration,the liquid was concentrated and subject to silica gel chromatograph. The column was eluted with DCM:MeOH = 30:1 to give Fmoc-Asn(NHNHCbz)- OtBu (3) as a white solid (250 mg,yield 89%). 1H NMR (400MHz, DMSO-d6):δ9.76 (s,1H),9.25 (s,1H),7.90 (d,2H,J = 7.5 Hz),7.72 (d, 2H,J = 7.5 Hz),7.60 (d,1H,J = 8.4 Hz),7.48-7.27 (m,9H),5.09 (s, 2H),4.39-4.16 (m,4H),2.61 (m,2H),1.38 (s,9H); HRMS (ESI-MS) calcd. for C31H33N3O7Na [M+Na]+ 582.2216,found 582.2218. A solution of Fmoc-Asn(NHNHCbz)-OtBu (3,6.2 g,10.6mmol) and TFA (38 mL) in DCM (152 mL) was stirred at room temperature for 3 h. The solventwas co-evaporatedwith toluene for three times. The resulted solidwas washed with ether to give Fmoc-Asn(NHNH2)-OH (4) as a white solid (3.1 g,yield 55%). 1H NMR (400 MHz,DMSO-d6): δ 9.80 (s,1H),9.24 (s,1H),7.90 (d,2H,J = 7.5 Hz),7.72 (d,2H, J = 7.5 Hz),7.54 (d,1H,J = 8.3 Hz),7.46-7.25 (m,9H),5.08 (s,2H), 4.46-4.33 (m,1H),4.32-4.14 (m,3H),2.65 (m,2H); 13C NMR (100 MHz,CD3OD):δ174.37,172.02,158.57,158.36,145.23, 145.21,142.51,137.64,129.47,129.13,128.98,128.75,128.17, 126.34,120.87,68.30,68.22,51.86,48.29,36.37; HRMS (ESI-MS) calcd. for C27H25N3O7Na [M+Na]+ 526.1590,found 526.1603.
Solid-phase peptide synthesis of Asn(NHNH2)-containing peptide: The peptides were synthesized on an automatic solidphase peptide synthesizer (CS Bio Co.) by the Fmoc-chemistry using Fmoc-protected amino acid derivatives. A 2-chlorotrityl resin was used as the solid support,in which the first amino acid (Thr) was attached through the ester linkage. To introduce a sidechain hydrazide residue at Asn,Fmoc-Asn(NHNHCbz)-OH (4) was used as the building block to replace the residue at Asn in the solidphase peptide synthesis. For synthetic cycles of normal amino acids,HATU (0.5 mol/L in DMF) and DIPEA (1.0 mol/L in DMF) (1:1, v/v) were used as the coupling reagent and piperidine (20% in DMF) was used as the deblocking reagent. For the synthetic cycle of Fmoc-Asn(NHNHCbz)-OH,the coupling reagent was replaced by DIC (40 mmol/L in DMF) and HOBT (40 mmol/L in DMF) (1:1,v/v) and the coupling reaction was prolonged for 12 h. Synthesis was carried out on a 0.2 mmol scale and 4-fold excess of Fmocprotected building blocks were used for each coupling reaction cycle. The peptides were cleaved from the resin by treatment with TFA/thioanisole/EDT/[3TD$DIF]anisole (90:5:3:2,v/v) followed by precipitation with cold ether. The crude peptide with Cbz group was treated with 5% trifluoromethanesulfonic acid (TFSA) in TFA to remove the Cbz on side-chain Asn hydrazide. The residue was subject to HPLC purification to give QYN(NHNH2)YST (7) in an overall yield of 35%. Analytic HPLC: tR = 8.2 min; HRMS (ESI-MS) calcd. for C34H47N9O13 Analytic HPLC: tR = 8.2 min; HRMS (ESI-MS) calcd. for C34H47N9O13 789.3293,found 790.3321 [M+H]+,812.3149 [M+Na]+, 829.2779 [M+K]+.
Branched peptide synthesis by side-chain native chemical
ligation: QYN(NHNH2)YST (7) (0.77 mmol/L in final concentration)
were added to the aqueous phosphate buffer (0.2 mol/L) containing
6.0 mol/L guanidinium chloride (Gn⋅HCl) and 0.8 mmol/L
benzamide as internal standard. At pH 3 and -10 °C,the oxidant
NaNO2 (10 mmol/L in final concentration) was added to the
mixture. HPLC monitoring and MS characterization indicated the
formation of peptide azide QYN(N3)YST (8) within 20 min. Analytic
HPLC: tR = 9.1 min; HRMS (ESI-MS) calcd. for C
3. Results and discussion
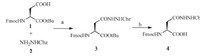
We sought to introduce a side-chain hydrazide on Asn during the Fmoc-based SPPS that requires a suitable synthetic building block with a fitted protecting group on hydrazine. Two optional strategies were on the table,TFA-labile protecting groups (Trt, BOC,etc.) or TFA-stable groups (Bn,Cbz,etc.). The advantage of TFA-labile groups is the simultaneous deprotection when using TFA-containing cleavage reagent to release the peptide from the resin. While,the late-stage deprotection of TFA-stable groups provide the compatibility with peptide C-terminus hydrazide,that enables the potential application of sequential hydrazide ligation at both C-terminal and side chain. Thus,we chose Cbz as the protecting group and synthesized the building block Fmoc- Asn(NHNHCbz)-OH (4) as shown in Scheme 2. Coupling of sidechain acid on Fmoc-Asp-OtBu (1) with Cbz-hydrazine (2) afforded Fmoc-Asn(NHNHCbz)-OtBu (3) in an excellent yield. Removal of tert-butyl on 3 by 20% TFA in dichloromethane gave the target building block 4 in a moderate yield.
|
Download:
|
| Scheme. 2.Synthesis of Fmoc-Asn(NHNHCbz)-OH. (a) HATU/DIPEA,89%; (b) 20% TFA in DCM,55%. | |
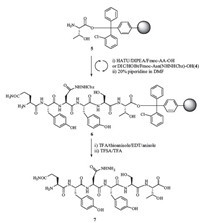
Next,we employed this building block to synthesize a sidechain hydrazide hexapeptide QYN(NHNH2)YST (7),derived from IgG Fc domain. As summarized in Scheme 3,the synthesis started on a Thr-preloaded 2-chlorotrityl resin. The initial attempt was carried out by normal coupling conditions under HATU and DIPEA for all amino acid cycles including the hydrazide building block 4. The poor yield of target peptide provoked us to investigate the synthesis. The major product lacked hydrazide Asn that implicated the problem of Asn cycle. It has been reported that glycosylated Asn building block could easily be self-cyclized between acid and amide forming an aspartimide under harsh coupling conditions [22]. Therefore,we exploited a mild coupling condition with DIC and HOBT in lower concentration (40 mmol/L) and prolonged the reaction time. Finally,we successfully accomplished the assembly of hydrazide Asn at more than 95% conversion rate. The rest cycles were continued and finished under normal coupling condition. Cleavage cocktail TFA/thioanisole/EDT/Anisole (90:5:3:2,v/v) was used to release the peptide and remove acid-labile protecting groups on side chains. The resulted peptide QYN(NHNHCbz)YST was treated with 5% TFSA in TFA for deprotection of Cbz group. The hydrazide peptide QYN(NHNH2)YST (7) was given in an overall yield of 35% counted from the starting amount of resin. HPLC (Fig. 1a,peak 1) and HRMS (Supporting information) characterization of 7 demonstrated the right compound was achieved in good purity. The found m/z of 790.3321 is in agreement with the calculated mass of 790.3372 for its formula.
|
Download:
|
| Scheme. 3.Solid-phase peptide synthesis of Gln-Tyr-Asn(NHNH2)-Tyr-Ser-Thr. | |

|
Download:
|
| Fig. 1.HPLC profiles of peptide side-chain ligation. (a) Peptide QYN(NHNH2)YST (peak 1) and internal standard (marked with *); (b) formation of acylazide QYN(N3)YST (peak 2) after 20 min with NaNO2; (c) thioester QYD(SAr)YST (peak 3) formation after 30 min with MPAA,peak 4 is the by-product of cyclization of Asp side chain to the nitrogen of neighbor Tyr; (d) side-chain ligation of the thioester with CKYMH (peak 5) after 24 h,peak 6 is the branched peptide QYN(CKYMH)YST. | |
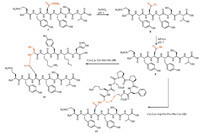
With the side-chain hydrazide peptide in hands,we were ready to investigate the side-chain ligation with N-terminus Cys peptide for synthesis of branched peptides. Firstly,the side-chain hydrazide needed to be activated to its thioester form following Liu’s approach [17,18]. Scheme 4,Figs. 1a-c and 2a,b described the procedures and simultaneous monitoring and characterization. The hydrazine was converted to acylazide by NaNO2 in a phosphate buffer containing guanidine (pH 3). The HPLC monitoring showed disappearance of 7 (Fig. 1a,peak 1) at the retention time of 8.2 min and a new peak (Fig. 1b,peak 2) emerged at the retention time of 9.1 min. The HRMS detection of peak 2 (Fig. 2a) displayed the m/z of 801.3145 that is highly in accordance with the calculated mass 801.3168 of the acylazide peptide QYN(N3)YST (8). The reaction of 8 and MPAA is extremely expeditious. After 2 min,HPLC profile showed the rapid conversion of 8 to its thioester form QYD(SAr)YST (9) (Fig. 1c,peak 3). The HRMS of 9 (Fig. 2b) validated the compound structure. During the thioester formation,we observed a tiny peak at 9.4 min and the HRMS (Fig. 2d) implicated it was the cyclization byproduct of cyclo-Asn with the neighbor Tyr nitrogen as reported in the references [20]. The basic condition and high temperature will accelerate the formation of cyclo-Asn byproduct. We have found the thioester is relatively stable under neutral pH 7 at 4 °C for a few days.
|
Download:
|
| Scheme. 4.Branched peptide synthesis by side-chain hydrazide ligation. | |

|
Download:
|
| Fig. 2.Mass spectra of branched peptides and intermediates. (a) QYN(N3)YST; (b) QYD(SAr)YST; (c) QYN(CKYMH)YST; (d) cyclo-Asn byproduct; (e) QYN(CKDPPFC)YST; (f) QYN[cyclo(CKDPPFC)]YST. | |
The synthesis of branched peptides by side-chain ligation between thioester on 9 and two Cys peptide CKYMH (10,a model peptide used in reference [23]) and CKDPPFC (12,a loop peptide of EGFR recognized by therapeutic antibody herceptin [yes])was carried out respectively under pH 7 at 4 °C. The ligation was monitored by HPLC and HRMS. Fig. 1d showed the ligation of 9 and10 after 24 h incubation. The peak 6 at 9.8 min was identified by HRMS (Fig. 2c) showing the found m/z 719.7944whichmatches the calculated twoproton charged mass of 719.7925 for formula C63H87N15O20S2 QYN(CKYMH)YST (11).Meanwhile,the cyclo-Asn byproduct (Fig. 1d, peak 4) also accumulated but slower than ligation during the reaction. After 48 h,the thioester was completely consumed and the branched peptide 11 was given in a moderate yield of 55% based on the calculation of consumption of 10 and internal standard-assisted quantification on 11 and cyclo-Asn byproduct.
In the end,we applied our method in preparation of a branched cyclic peptide by ligation of 9 and Cys-peptide 12 containing two Cys residues for late-stage disulfide bond formation. The HPLC monitoring of the ligation (Supporting information) detected a newly emerging peak of branched peptide QYN(CKDPPFC)YST with two free Cys residues and the HRMS data (Fig. 2e) supported the identification. The disulfide bond formation by oxidation with dimethyl sulfoxide and air gave the branched cyclic peptide QYN[cyclo(CKDPPFC)]YST in an overall isolated yield of 50%. The found m/z of 782.8075 in HRMS (Fig. 2f) verified the disulfide structure. This approach provides a novel and efficient synthesis of branched cyclic peptide that facilitates the design of neopeptide as mimics of functional protein domains. Its potential application on sequential ligation at both C-terminal and side chain would facilitate the synthesis of longer branched peptide.
4. Conclusion
A new method for synthesis of branched peptide by side-chain hydrazide ligation was successfully achieved. An Asn side-chain hydrazide was incorporated into a peptide in SPPS as the precursor of thioester. After converted from hydrazide to thioester by NaNO2 and thiol reagent,the side-chain ligation with N-terminus Cyspeptides was carried out to afford respective branched peptides efficiently. The introduced two-Cys-peptide formed a disulfide bond to give the branched cyclic peptide. This approach facilitates the synthesis of branched peptides for functional bio-mimics development.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NNSFC,No. 21372238),National 1000-Young-Talent Program of China,and Shanghai Pujiang Talent Program (No. 13PJ1410200). We thank the members in Huang’s group for helpful discussion and technical support.
| [1] | G.J. Gorse, M.C. Keefer, R.B. Belshe, et al., A dose-ranging study of a prototype synthetic HIV-1MN V3 branched peptide vaccine. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group, J. Infect. Dis. 173 (1996) 330-339. |
| [2] | N. Yahi, J.M. Sabatier, S. Baghdiguian, F. Gonzalez-Scarano, J. Fantini, Synthetic multimeric peptides derived from the principal neutralization domain (V3 loop) of human immunodeficiency virus type 1 (HIV-1) gp120 bind to galactosylceramide and block HIV-1 infection in a human CD4-negative mucosal epithelial cell line, J. Virol. 69 (1995) 320-325. |
| [3] | A. Benjouad, F. Chapuis, E. Fenouillet, J.C. Gluckman, Multibranched peptide constructs derived from the V3 loop of envelope glycoprotein gp120 inhibit human immunodeficiency virus type 1 infection through interaction with CD4, J. Virol. 206 (1995) 457-464. |
| [4] | J.M. Sheridan, G.M. Hayes, B.M. Austen, Solid-phase synthesis and cyclization of a large branched peptide from IgG Fc with affinity for Fc γRI, J. Pept. Sci. 5 (1999) 555-562. |
| [5] | A. Stavrakoudis, S. Makropoulou, V. Tsikaris, et al., Computational screening of branched cyclic peptide motifs as potential enzyme mimetics, J. Pept. Sci. 9 (2003) 145-155. |
| [6] | D. Li, D.L. Elbert, The kinetics of the removal of the N-methyltrityl (Mtt) group during the synthesis of branched peptides, J. Pept. Res. 60 (2002) 300-303. |
| [7] | S.A. Kates, S.B. Daniels, F. Albericio, Automated allyl cleavage for continuousflow synthesis of cyclic and branched peptides, Anal. Biochem. 212 (1993) 303-310. |
| [8] | D. Delforge, M. Art, B. Gillon, et al., Automated solid-phase synthesis of cyclic peptides bearing a side-chain tail designed for subsequent chemical grafting, Anal. Biochem. 242 (1996) 180-186. |
| [9] | G.B. Bloomberg, D. Askin, A.R. Gargaro, M.J.A. Tanner, Synthesis of a branched cyclic peptide using a strategy employing Fmoc chemistry and two additional orthogonal protecting groups, Tetrahedron Lett. 34 (1993) 4709-4712. |
| [10] | S.W. Millward, H.D. Agnew, B. Lai, et al., In situ click chemistry: from small molecule discovery to synthetic antibodies, Integr. Biol. (Camb.) 5 (2013) 87-95. |
| [11] | Y.J. Pu, H. Yuan, M. Yang, B. He, Z.W. Gu, Synthesis of peptide dendrimers with polyhedral oligomeric silsesquioxane cores via click chemistry, Chin. Chem. Lett. 24 (2013) 917-920. |
| [12] | K.K. Pasunooti, R. Yang, S. Vedachalam, et al., Synthesis of 4-mercapto-L-lysine derivatives: potential building blocks for sequential native chemical ligation, Bioorg. Med. Chem. Lett. 19 (2009) 6268-6271. |
| [13] | D. Li, D.L. Elbert, Unprotected peptides as building blocks for branched peptides and peptide dendrimers, J. Pept. Res. 60 (2002) 300-303. |
| [14] | J.A. Camarero, A.R. Mitchell, Synthesis of proteins by native chemical ligation using Fmoc-based chemistry, Protein Pept. Lett. 12 (2005) 723-728. |
| [15] | B.L. Nilsson, M.B. Soellner, R.T. Raines, Chemical synthesis of proteins, Annu. Rev. Biophys. Biomol. Struct. 34 (2005) 91-118. |
| [16] | Q.Q. He, G.M. Fang, L. Liu, Design of thiol-containing amino acids for native chemical ligation at non-cys sites, Chin. Chem. Lett. 24 (2013) 265-269. |
| [17] | P. Wang, X. Li, J. Zhu, et al., Encouraging progress in the omega-aspartylation of complex oligosaccharides as a general route to beta-N-linked glycopolypeptides, J. Am. Chem. Soc. 133 (2011) 1597-1602. |
| [18] | S.T. Cohen-Anisfeld, P.T. Lansbury, A practical, convergent method for glycopeptide synthesis, J. Am. Chem. Soc. 115 (1993) 10531-10537. |
| [19] | J.S. Zheng, S. Tang, Y.C. Huang, L. Liu, Development of new thioester equivalents for protein chemical synthesis, Acc. Chem. Res. 46 (2013) 2475-2484. |
| [20] | G.M. Fang, Y.M. Li, F. Shen, et al., Protein chemical synthesis by ligation of peptide hydrazides, Angew. Chem. Int. Ed. Engl. 50 (2011) 7645-7649. |
| [21] | J.S. Zheng, S. Tang, Y.K. Qi, Z.P. Wang, L. Liu, Chemical synthesis of proteins using peptide hydrazides as thioester surrogates, Nat. Protocols 8 (2013) 2483-2495. |
| [22] | N. Yamamoto, A. Tkayanagi, A. Yoshino, T. Sakakibara, Y. Kajihara, An approach for a synthesis of asparagine-linked sialylglycopeptides having intact and homogeneous complex-type undecadisialyloligosaccharides, Chem. Eur. J. 13 (2007) 613-625. |
| [23] | H.S. Cho, K. Mason, K.X. Ramyar, et al., Structure of the extracellular of HER2 alone and in complex with the Herceptin Fab, Nature 421 (2003) 756-760. |




