Ionic liquids (ILs) have gained increasing concerns due to low melting point,good chemical stability and easiness to be functionalized such as catalysis [1]. In recent few years,polymer ionic liquids (PILs) as novel polyelectrolytes are becoming more attractive [2]. It is worthy to note that PILs can derive a huge family of functional materials after substitution of the corresponding anions or cations [3]. For instance,the imidazole based ionic liquids can experience a reversible affinity transition in between hydrophobic-hydrophilic by simply exchanging anions [4]. Wettability of the IL or PIL coating is thus simply tunable from hydrophilic to hydrophobic when the anion BF4- is substituted with PF6- group [5]. Besides controlling wettability,PILs will possess excellent redox-catalytic activity after the corresponding functional anions such as polyoxometalates (POMs) are introduced [6]. The catalytic PILs are promising in decomposition of water pollutants [7]. Among the pollutants,those water soluble azobased dyestuffs are commonly used but toxic to environment. It is important to develop methods to effectively decompose the dyestuffs. Oxidation process [8] is more efficient than other methods including physical adsorption process [9] and biological method [10]. Polyoxometalates (POMs) are a series of molecular metal-oxygen clusters with varied structures [11]. Especially, those polyoxometalates with a Keggin structure such as H3PW12O40 have shown excellent redox-catalytic activity in the decomposition of azo dyes [6]. It is expected that POMs based IL or PIL will be capable to catalytic degradation of dyes. It becomes a key concern to support the catalysts for easy recycling after the decomposition process. We have recently reported on synthesis of bamboo-like crosslinked polymer nanotubes via a rapid cationic polymerization [12]. Compared with previously reported methods such as self-assembly,template synthesis and electrospinning [13],the method is highly effective to synthesize the nanotubes in large scale.
Herein,we report on the synthesis of PW12O40 3- based PIL functionalized polymer nanotubes (Scheme 1) and decomposition of the azo-based dyestuffs. Poly(divinylbenzene-co-vinylbenzyl chloride) (DVB-co-VBC) nanotubes are synthesized. The poly(VBC) block can further initiate ATRP to graft IL monomer of ViEtIm+Br- onto the polymer nanotubes. After a simple anion substitution of Br- with PW12O40 3-,PW12O40 3- based PIL functionalized poly(DVB-co-VBC) nanotubes are synthesized. The composite nanotubes are dispersed in aqueous solution well,which can catalyze decomposition of water soluble dyes. Compared with IL molecules and their polymeric PILs,the PIL modified nanotubes can be easily collected from water for separation and regeneration.

|
Download:
|
| Scheme. 1.Schematic synthesis of the PW12O403- based PIL composite nanotubes. Poly(DVB-co-VBC) nanotubes are fabricated by cationic polymerization of DVB and VBC monomers (a). Br- based PIL composite nanotubes are synthesized by ATRP grafting of [(ViEtIm)+Br-] from the poly(DVB-co-VBC) nanotubes surface (b).PW12O403- based PIL composite nanotubes are synthesized by anion exchange of Br- with PW12O403- (c). | |
Divinyl benzene (DVB),vinylbenzyl chloride (VBC) and 1,1,4,7,7- pentamethyldiethylenetriamine(PMEDTA) were purchased from Sigma-Aldrich. Boron trifluoride diethyl etherate (BF3O(Et)2,CP), ethanol (AR),n-hexane (AR) were supplied by Beijing Chemical Works. The example dye methyl orange (MO) was purchased from Sinopharm Chemical Reagent Beijing. The ionic liquid monomer of 1-vinyl-3-ethylimidazolium bromide [(ViEtIm)+Br-] was synthesized [5].
2.2. Synthesis of the poly(DVB-co-VBC) nanotubesAt room temperature,e.g. 25 °C,150 mg of BFEE was immediately added into 150 μL of heptane under ultrasonication. A given amount of DVB and VBC mixture (3/2,v/v) was added to start the polymerization under ultrasonication. In order to monitor growth of the polymer nanotubes at different stages,10.0 g of ethanol was added to terminate the polymerization. The samples were filtered and washed with ethanol to remove residual initiator and monomer.,
Synthesis of the Br- based PIL composite nanotubes: 20.0 mg of poly(DVB-co-VBC) nanotubes,12.0 μL of PMEDTA,1.0 g of [(ViEtIm)+Br-] were mixed in 6.0 μL of methanol. The mixture was degassed by freeze thawing. After 6.0 mg of CuBr was added, the green mixture was sealed under vacuum. After the ATRP reaction was performed at 70 °C for 12 h,the product was washed with methanol and centrifugated. The Br- based PIL composite nanotubes were obtained.
2.3. Synthesis of the PW12O40 3- based PIL composite nanotubesAfter 20.0 mg of the Br- based PIL composite nanotubes was dispersed in 20.0 μL of water,60.0 mg of H3PW12O40 was added. The system stood under stirring at room temperature for 1 h to allow the anion exchange. The PW12O40 3- based PIL composite nanotubes were obtained after centrifugation and wash with water.
2.4. Catalytic decomposition of MO 5.0 mg of PW12O403- based PIL composite nanotubes was dispersed in 2.0 μL of methyl orange (MO) aqueous solution (20.0 mg L-1). After 10.0 μL of H2O2 (30 wt%) was added under stirring at room temperature,the MO solution was centrifugated from the reaction system at a given interval. The characteristic absorption band at 463 nm of MO is used to evaluate removal degree calculated by (C0 - C)/C0,while C0 is the original MO concentration,C is the MO concentration after the decomposition time.
2.5. CharacterizationMorphology of the samples was characterized via scanning electron microscopy (SEM) characterization (HITACHI S-4800 operating at 15 kV) and transmission electron microscopy (TEM) characterization (JEOL 100CX operating at 100 kV). The samples for SEM observation were prepared by vacuum sputtering with Pt after being ambient dried. FT-IR spectroscopy was performed after scanning the samples for 32 times using a Bruker EQUINOX 55 spectrometer with the sample/KBr pressed pellets. UV-vis spectroscopy was performed using UV spectrophotometer (TU1901).
3. Results and discussionAs previously reported [12],PDVB bamboo-like nanotubes are easily fabricated within minutes by cationic polymerization using immiscible initiator nanodroplets of BFEE at room temperature. In our current concern,vinylbenzyl chloride (VBC) is used to copolymerize with DVB forming the corresponding poly(DVB-co- VBC) nanotubes (Fig. 1a). The polymerization is completed within 5 min. Bamboo-like segmental cavity is clearly discerned with a periodic diaphragm about 500 nm. The nanotubes are ∼150 nm in diameter,whose surface is smooth (Fig. 1b). Presence of VBC is confirmed by FT-IR spectrum (Fig. 1c). The characteristic peaks at 671 cm-1 and 1264 cm-1 are assigned to -CH2-Cl of DVB in the polymer nanotubes. EDX result reveals the presence of Cl element (Fig. 1d). The benzyl chloride group onto the poly(DVB-co-VBC) nanotubes can be used as an agent to initiate ATRP grafting of other polymers from the nanotube surface. An IL monomer of 1-vinyl-3- ethylimidazolium bromide (ViEtIm+Br-) is selected for the polymerization. Presence of the imidazolecation is confirmed by the bands at 1700-1750 cm-1 in FT-IR spectrum (Fig. 1e). The characteristic peaks at 1465 cm-1 is assigned to C-N. EDX result indicates the presence of N and Br elements at the PIL composite nanotubes (Fig. 1f).

|
Download:
|
| Fig. 1.(a and b) TEM and SEM images of the poly(DVB-co-VBC) nanotubes; (c) FT-IR spectrum and (d) EDX spectrum of the nanotubes. DVB/VBC monomer volume ratio is 3:2. During the synthesis,the mixed monomer concentration is fixed at 2.5 wt%,polymerization time is 5 min; (e) FT-IR spectrum and (f) EDX spectrum of the Br- based PIL composite nanotubes after ATRP grafting from the poly(DVB-co-VBC) nanotubes. | |
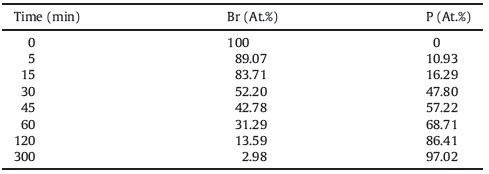
Anion of PILs can be easily exchanged to render new performances. After Br- is exchanged with PW12O40 3-,PW12O40 3- based PIL composite nanotubes are derived. The smooth surface and bamboo-like structures are well preserved (Fig. 2a and b). While Br element content is decreased,P content is increased accordingly with prolonging anion exchange time (Table 1). When the anion exchange time is 1 h,Br element is dramatically decreased from the original 1.88% to 0.51% after the anion exchange while the composite nanotubes contain 1.12% of P element and 12.0% of W element (Fig. 2c). The as-prepared PW12O40 3- based PIL composite nanotubes are well dispersed in water (right,Fig. 2d). In contrast,the poly(DVB-co-VBC) nanotubes are not dispersible in water (left,Fig. 2d). The dispersibility enhancement in water will greatly facilitates a further catalytic decomposition of water soluble dyes.
| Table 1 EDX data of Br and P contents of the PW12O403- based PIL composite nanotubes with prolonging anion exchange time. |

|
Download:
|
| Fig. 2.PW12O403- based PIL composite nanotubes. (a) TEM and (b) SEM images of the composite nanotubes; (c) EDX spectrum of the composite nanotubes; (d) aqueous dispersions of the poly(DVB-co-VBC) nanotubes (left) and PW12O403- based PIL composite nanotubes (right). | |
PW12O40 3- is highly effective in catalytic heterogeneous decomposition of organic dyes. MO is selected as a model water soluble organic dye. The MO aqueous solution is orange in color (left,Fig. 3a),which becomes colorless (right,Fig. 3a) after decomposition with the PW12O40 3- based PIL composite in the presence of 10.0 μL of H2O2 at room temperature after 0.5 h. In comparison,the colorful solution is less influenced when H2O2 is not added. The characteristic absorption band at 463 nm of MO is used to evaluate removal degree from the MO solution (curve 1,Fig. 3b). As shown in curve 2 (Fig. 3b),very strong characteristic absorption peak of MO is preserved,which is independent on time. This indicates that the dye absorption by the composite nanotubes is dominant. 49.5 wt% of MO is removed. When H2O2 is added,78.7 wt% of MO is removed after 0.5 h (curve 3,Fig. 3b). This implies that introduction of H2O2 can greatly facilitate the decomposition. After prolonging time to 1 h,96.5 wt% of MO is removed (curve 4,Fig. 3b). This indicates that the catalytic decomposition is dominant. It is well known that more active peroxides such as [(PO4){WO(O2)2}2{WO(O2)2(H2O)}]3- and [(PO4){WO(O2)2}4]3- are derived from PW12O40 3- after adding H2O2 [14]. On the other hand,the intermediate products are hydrophobic,which can be easily transferred into the hydrophobic internal sites of the composite nanotubes. The decomposition is thus greatly enhanced. The composite nanotubes can be separated from the system by either centrifugation or filtration after decomposition of the dyes. The composite nanotubes are thus simply recycled.

|
Download:
|
| Fig. 3.(a) MO aqueous solution before (left) and after (right) catalytic degradation by the PW12O403- based composite nanotubes in the presence of 10.0 μL ofH2O2; (b) UV- vis spectra of 20.0 ppm of MO aqueous solution (curve 1); MO concentration after treatment with the PW12O403- based composite nanotubes after 2 h in the absence of H2O2 (curve 2); MO concentration after decomposition in the presence of 10.0 μL of H2O2 after 0.5 h (curve 3) and after 1 h (curve 4). | |
In summary,we have proposed an efficient approach to large scale synthesize poly(DVB-co-VBC) nanotubes by cationic polymerization of DVB and VBC monomer mixture. PIL functionalized polymer nanotubes are fabricated by grafting IL monomers for example ViEtIm+Br- from the nanotubes surface via ATRP. By anion exchanging of Br- with PW12O40 3-,the PW12O40 3- composite nanotubes are derived which can effectively decompose an example water soluble dye MO in the presence of H2O2. Higher decomposition efficiency is resulted from the cooperative interplay between the synchronous decomposition gradation and phase transfer of the hydrophobic intermediate products. The catalytic nanotubes are easily separated by simple filtration for recycling.
AcknowledgmentsThis work was supported by MOST (No. 2012CB933200) and NSFC (Nos. 51233007 and 51173191).
| [1] | (a) S. Keskin, D. Kayrak-Talay, U. Akman, A review of ionic liquids towards supercritical fluid applications, J. Supercrit. Fluids 43 (2007) 150-180; (b) H. Niedermeyer, J.P. Hallett, I.J. Villar-Garcia, P.A. Hunt, T. Welton, Mixtures of ionic liquids, Chem. Soc. Rev. 41 (2012) 7780-7802; (c) S. Karimian, H. Tajik, N-protection of amines using pyridinium 2,2,2-trifluoroacetate ionic liquid as an efficient and reusable catalyst, Chin. Chem. Lett. 25 (2014) 218-220; (d) F. Shirini, A. Yahyazadeh, K. Mohammadi, One-pot synthesis of various xanthene derivatives using ionic liquid 1,3-disulfonic acid imidazolium hydrogen sulfate as an efficient and reusable catalyst under solvent-free conditions, Chin. Chem. Lett. 25 (2014) 341-347. |
| [2] | (a) H.L. Ricks-Laskoski, A.W. Snow, Synthesis and electric field actuation of an ionic liquid polymer, J. Am. Chem. Soc. 128 (2006) 12402-12403; (b) W. Lu, G.A. Fadeev, B. Qi, et al., Use of ionic liquids for pi-conjugated polymer electochemical devices, Science 297 (2002) 983-987. |
| [3] | (a) X.B. Hu, J. Huang, W.X. Zhang, et al., Photonic ionic liquids polymer for nakedeye detection of anions, Adv. Mater. 20 (2008) 4074-4078; (b) F.R. Tao, C. Zhuang, Y.Z. Cui, J. Xu, Dehydration of glucose into 5-hydroxymethylfurfuralin SO3H-functionalized ionic liquids, Chin. Chem. Lett. 25 (2014) 757-761. |
| [4] | (a) B. Yu, F. Zhou, G. Liu, et al., The electrolyte switchable solubility of multiwalled carbon nanotube/ionic liquid (MWCNT/IL) hybrids, Chem. Commun. (2006) 2356-2358; (b) X.Y. He, W. Yang, X.W. Pei, Preparation, characterization, and tunable wettability of poly(ionic liquid) brushes via surface-initiated atom transfer radical polymerization, Macromolecules 41 (2008) 4615-4621. |
| [5] | X.Y. Ji, Q. Zhang, F.X. Liang, et al., Ionic liquid functionalized Janus nanosheets, Chem. Commun. 50 (2014) 5706-5709. |
| [6] | C. Ritchie, C. Streb, J. Thiel, et al., Reversible redox reactions in an extended polyoxometalate framework solid, Angew. Chem. Int. Ed. Engl. 47 (2008) 6881-6884. |
| [7] | (a) O. Legrini, E. Oliveros, A.M. Braun, Photochemical processes for water treatment, Chem. Rev. 93 (1993) 671-698; (b) M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahnemannt, Environmental applications of semiconductor photocatalysis, Chem. Rev. 95 (1995) 69-96. |
| [8] | I.M. Arabatzisa, T. Stergiopoulosa, D. Andreevab, et al., Characterization and photocatalytic activity of Au/TiO2 thin films for azo-dye degradation, J. Catal. 220 (2003) 127-135. |
| [9] | G. Annadurai, R.S. Juang, D.J. Lee, Use of cellulose-based wastes for adsorption of dyes from aqueous solutions, J. Hazard. Mater. 92 (2002) 263-274. |
| [10] | C.T.M.J. Frijters, R.H. Vos, G. Scheffer, R. Mulder, Decolorizing and detoxifying textile wastewater, containing both soluble and insoluble dyes, in a full scale combined anaerobic/aerobic system, Water Res. 40 (2006) 1249-1257. |
| [11] | Y.P. Jeannin, The nomenclature of polyoxometalates: how to connect a name and a structure, Chem. Rev. 98 (1998) 51-76. |
| [12] | W. Ni, F.X. Liang, J.G. Liu, et al., Polymer nanotubes toward gelating organic chemicals, Chem. Commun. 47 (2011) 4727-4729. |
| [13] | (a) M.F. De Volder, H. Tawfick, R.H. Baughman, A.J. Hart, Carbon nanotubes: present and future commercial applications, Science 339 (2013) 535-539; (b) K. Lee, A. Mazare, P. Schmuki, One-dimensional titanium dioxide nanomaterials: nanotubes, Chem. Rev. 114 (2014) 9385-9454. |
| [14] | D.C. Duncan, R.C. Chambers, E. Hecht, C.L. Hill, Mechanism and dynamics in the H3[PW12O40]-catalyzed selective epoxidation of terminal olefins by H2O2. Formation, reactivity, and stability of {PO4[WO(O2)2]4}3-, J. Am. Chem. Soc. 117 (1995) 681-691. |