b Beijing National Laboratory for Molecular Sciences, State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
Metal salen/salophen complexes (salen= N,N'-bis(salicylidene)ethylenediaminato; salophen = N,N0-bisalicylidene-1,2-phenylenediaminato) have been extensively studied for their potential applications in catalysis [1],optics materials [2] and biological metalloenzyme mimics [3]. Among them,ZnII complexes,firstly discovered by Pfeiffer in 1933,represent an important class of metallosalens. These complexes can be easily prepared by condensation of salicylaldehyde and diamine in refluxing alcohols. More importantly,their absorption and emission could be tuned from blue to red by modification of the conjugated spacers,the bridge connecting the two nitrogen donor atoms and the substituents in salicylaldehyde. In past decades,Znsalens have been proved to be excellent candidates for the development of new luminescent materials in organic light-emitting diode devices [4]. Recently,we and others extended their application to biological studies such as DNA/RNA binding,biomolecular sensors and cell imaging,which enriches the realm of Znsalen chemistry DNA/RNA binding [5],biomolecular sensors [6] and cell imaging [7]. Particularly,for the low cytotoxicity,effective cellular-uptake and high luminescence applicable in one or two photon fluorescence microscopy,Znsalen and its derivative became emerging luminescent metal reagents in cell imaging [8]. Thus, deeper understanding of the relationship between luminescence and structures for Znsalens is of importance to further design functional materials and imaging reagents with high luminescence. Despite of ligand-dependent fluorescence arising from close-shell d10 Zn ion,another feature for Znsalens is Lewis acidicity of Zn2+ ion with planar structure. Besides the dipolar or p-p stacking interactions often observed in perylenebisimide dyes or porphyrins,Zn ion has tendency to pentacoordination,which allows the axial interaction of coordinating species such as phosphate anions,solvents or intermolecular phenolic O…Zn axial coordination. Switching from ‘‘deaggregation (monomeric species with coordination with solvents or Lewis bases) to aggregation (intermolecular O…Zn axial coordination)’’ is accompanied with the turn-on to -off fluorescence. This attractive property renders Znsalens the useful synthons for supramolecular architecture with potential applications in catalysis [9],sensors [10] and optical materials [11]. However,an obvious disadvantage of aggregation induced by intermolecular O…Zn axial coordination is the low solubility and quenched luminescence in the solution of non-coordinating solvents such as CH2Cl2,toluene and n-hexane [12]. Thus,for the purpose of OLED and even biological application,how to minimize the self-assembly of Znsalens is an important issue. Toward this goal,tremendous progress made in addition of Lewis bases to disassociate Znsalen aggregates [13], however,suffering from the low binding affinity of Lewis bases (ca. <104 mol/L). Thus,increasing steric bulky of salen skeleton becomes an alternative approach. Recently,Nabeshima et al. reported a steric bulky and robust tris(Znsalen) crytpand which exhibits unique binding behavior to MOAc (M = Na,K,Rb,Cs) as cation guests [14]. However,the photophysical properties of such tris(Znsalen) complex have not been well studied. We are interested in the effect of rigid and robust structure on the luminescence and envisioned that the orientation of Znsalen units in such crytand structures may prevent Znsalen aggregation arising from intermolecular Zn…O interaction.
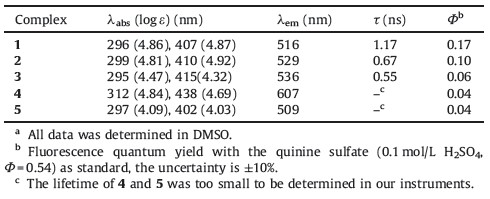
In this work,we synthesized and characterized four tris(Znsalen) cryptands (1-4) with different substituted diamines, o-phenyldiamine,4,5-bis-(ethoxycarbonylmethoxy)benzene-1,2- diamine,3,4,5,6-tetrafluoro-1,2-phenylenediamine and 4,5- diamino-1,2-benzenedicarbonitrile (Fig. 1) and complex 1 displays the highest fluorescence in dimethyl sulfoxide (DMSO) among the four complexes. To demonstrate the effect of cryptand structure on the Znsalen aggregation,we chose monomer Znsalen 5 as control to investigate the photophysical properties of complex 1 in the solution of non-coordination solvent CH2Cl2 (DCM) and found that Znsalen 1 could minimize the self-assembly through intermolecular Zn…O interaction. More importantly,to demonstrate the potential application in biological study,we prepared 1 and 5 loaded poly(lactide-co-glycolide) nanoparticles (PLGA NPs). Cell imaging experiments showed that 1 loaded PLGA NPs (NPs-1) have clear intracellular fluorescence emission while 5 loaded PLGA NPs (NPs-5) showed negligible fluorescence,which might be due to the more aggregated form of 5 inside the PLGA-NPs. Thus,given that the well established cryptand chemistry,the combination of luminescent metal complexes would provide a new access to design new luminescent materials with the potential application in optics and biological studies.
|
Download:
|
| Fig. 1.Chemical structures of Znsalen complexes. | |
All solvents and chemicals were purchased from Alfa Aesar and J&K and were used without further purification,unless specifically mentioned,PLGA (MW = 10,000) was purchased from Daigang Biomaterial Co.,Ltd.,Jinan,China,cellular imaging trackers were purchased from Invitrogen (Life Technologies). 4,5-Bis-(ethoxycarbonylmethoxy)- benzene-1,2-diamine (diamine A2) was synthesized by a modified literature method [15]. 3,4,5,6-Tetrafluoro- 1,2-phenylenediamine (diamine A3) was synthesized according to the literature method [16]. Trialdehyde (3,3',3''-methanetriyltris( 2-hydroxybenzaldehyde)) was synthesized according to the procedure reported by Nabeshima and coworkers [14]. The 1HNMR spectroscopic measurements were recorded on a Bruker-400 NMR spectrometer using TMS as internal standard. Electrospray ionization (ESI) mass spectra were performed on a Fourier Transform Ion Cyclotron Resonance Mass Spectrometer (FT-ICR,Bruker,USA). FT-IR spectra were taken on a Nicolet iN10 MX Fourier Transform Infrared Spectrometer. The UV-vis absorption spectra were recorded on an Agilent 8453,UV-vis spectrophotometer in 1 cm path length quartz cells. Fluorescence spectra were recorded on a lifetime and steady state spectrophotometer (Edinburgh Instrument FLS920). Quantum yields of one photon emission of complexes 1-5 were measured in DMSO relative to the fluorescence of quinine sulfate in 0.1 mol/L H2SO4 (Flum = 0.54). Confocal fluorescent images of living cells were performed using Nikon A1R-Si. Laser Scanning Confocal Microscope (Japan),equipped with the laser of 405 nm.
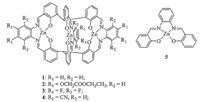
General synthesis of tri(Znsalen) complexes 1-4: The mixture of (3,3',3''-methanetriyltris(2-hydroxybenzaldehyde)) (75.2 mg, 0.20 mmol,2 eq.),diamine A1-4 (0.3 mmol,3 eq.) and Zn(OAc)2.2H2O (68.0 mg,0.31 mmol,3.1 eq.) with were refluxed in acetonitrile/chloroform (2 mL/2 mL) for 2 days (Scheme 1) [14]. After cooling to the room temperature,the precipitates were filtered and washed with acetonitrile,then dried under vacuum to give the desired complexes 1-4. Complex 1 (72 mg,yield 62%): 1H NMR (400 MHz,DMSO-d6):δ8.53 (s,6H),7.50-7.53 (m,8H),7.30 (dd,6H),7.15 (dd,6H,J = 7.6,1.8 Hz),6.80 (dd,6H,J = 7.6,1.8 Hz), 6.37 (t,6H,J = 7.6 Hz). FT-IR (KBr pellete,cm-1): 1616 (C≡N),1537 (Ar C≡C),1435 (Ar C≡C),1186 (C-O). HR MS (ESI+,DMSO,FT-ICR): m/z calcd. for C68H57N6O9S3Zn3 ([M+3DMSO+H]+) 1389.1218, found 1389.1239. Complex 2 (92 mg,yield 52%): 1H NMR (400 MHz,DMSO-d6):δ8.47 (s,6H),7.52 (s,2H),7.21 (s,6H), 7.10 (dd,6H,J = 7.4 Hz),6.78 (dd,6H,J = 7.4 Hz),6.36 (t,6H, J = 7.4 Hz),4.91 (d,12H),4.15-4.21 (m,12H),1.24 (t,18H,). FT-IR (KBr pellete,cm-1): 1747(C≡O),1616 (C≡N),1539 (Ar C≡C),1430 (Ar C≡C),1173 (C-O). HR MS (ESI+,DMSO,FT-ICR): m/z calcd. for C86H74N6NaO24Zn3 ([M+Na]+) 1789.2521,found 1789.2527. Complex 3 (76 mg,yield 55%): 1H NMR (400 MHz,DMSO-d6): d 8.68 (s,6H),7.28 (s,2H),7.16 (dd,6H,J = 7.3 Hz),6.81 (dd,6H, J = 7.3 Hz),6.42 (t,6H,J = 7.3 Hz). FT-IR (KBr pellete,cm-1): 1605 (C≡N),1552 (Ar C≡C),1431 (Ar C≡C),1193 (C-O). HR MS (ESI+, DMSO,FT-ICR): m/z calcd. for C64H33F12N6O7SZn3 ([M+DMSO+H]+) 1448.9809,found 1448.9776. Complex 4 (74 mg,yield 57%): 1H NMR (400 MHz,d6-DMSO):δ8.68 (s,6H),8.33 (s,6H),7.43 (s,2H), 7.19 (dd,6H,J = 7.5 Hz),6.83 (dd,6H,J = 7.5 Hz),6.44 (t,6H, J = 7.5 Hz). FT-IR (KBr pellete,cm-1): 2229.8 (CBN),1612 (C≡N), 1541(Ar C≡C),1425 (Ar C≡C),1178 (C-O). HR MS (ESI+,DMSO,FTICR): m/z calcd. for C70H39N12O7SZn3 ([M+DMSO+H]+) 1387.0653,found 1387.0663.
|
Download:
|
| Scheme. 1.Synthetic route for complexes 1-4. | |
Synthesis of NPs-1 and NPs-5: 1 and 5 loaded PLGA-NPs were prepared by the modified literature method [17], 1 mmol 1 or 5 and 10 mg of PLGA were dissolved into 1 mL DCM. The DCM solution was added dropwise to 10 mL of aqueous solution rotating at 5000 rpm containing poly(vinyl alcohol) (PVA,2.5 wt% in ultrapure water; MW= 30,000-70,000 Da from Sigma-Aldrich) as a surfactant, sonicated for 40 s under ice-bath and then stirred overnight to evaporate the DCM. After removing large particles by centrifugation at 5000 rpm for 30 min,the PLGA NPs were collected by ultracentrifugation at 19,000 rpm for 30 min and washed with water three times to remove excess PVA. The washed PLGA NPs were filtered with a 0.45 mm membrane filter before use.
DMSO/DCM titration experiment: To a 2 mL of 0.05 mmol/L solution of 5 in DCM,accumulative total amount of 5,10,20,50, 100,200,500,1000,1500 eq. DMSO was added and the corresponding UV-vis absorption spectra and fluorescent emission spectra were recorded at 298 K. Titration experiment of complex 1 (0.025 mmol/L) with DMSO was conducted in a similar procedure.
3. Results and discussion3.1. Synthesis and characterization
In this work,we chose four different substituted phenyl diamines,o-phenylenediamine,4,5-bis(ethoxycarbonylmethoxy)- benzene-1,2-diamine,3,4,5,6-tetrafluoro-1,2-phenylenediamine and 4,5-diamino-1,2-benzenedicarbonitrile to investigate the electronic effect on the photophysical properties of tris(Znsalen) cryptand complexes. Complexes 1-4 were synthesized according to literature procedures [14] with slight modification. We used one-pot condensation of the trialdehyde (3,3',3''-methanetriyltris( 2-hydroxybenzaldehyde)),diamine,and Zn(OAc)2.2H2O in a ratio of 2:3:3 in the refluxing mixed solution of MeCN/CHCl3 afforded the desired complexes in the yields of 62%,52%,55% and 57%,respectively. The synthesis of the trialdehyde,diamine for 2, and the tris(Znsalen) crytand complexeswas shown in Scheme 1. Mononuclear Znsalen 5 was also synthesized by one pot reaction of salicylaldehyde,o-phenylenediamine and Zn(OAc)2 in refluxing ethanol [18]. Complexes 1-5 were characterized by 1H NMR, ESI-MS,IR and UV-vis spectroscopes and meters (details are given in the Supporting information). IR spectra of the compounds 1-4 containing aldimine moiety usually exhibit sharp bands at 1616,1616,1605,1612 and 1615 cm-1, respectively. The band due to νC=N in 3 exhibited a noticeable shift of 13 cm-1 relative to 1 and 2 and vibrated at 1605 cm-1. A decrease in the stretching frequency associated with νC=N in 3 indicated the electronic withdrawing effect of the substituent of diamines. In 1H NMR spectrum,the singlet for the characterized imine CH≡N protons were 8.53,8.47,8.68 and 8.68 ppm for 1-4, and the down-shifted 0.15 ppmof the CH≡N protons of complex 4 compared to 1,indicating the electron-withdrawing capacity of the diamines. The ESI-MS strongly supported formation of tris(Znsalen) cryptand complexes. In HR ESI-MS spectra of 1-4 displayed molecular ion peaks at m/z 1389.1239 ([M+3DMSO+H]+), 1789.2521 ([M+Na]+),1448.9776 ([M+DMSO+H]+),1387.0663 ([M+DMSO+H]+),which were consistent to the calculations. 3.2. Photophysical properties
The absorption and emission spectra of complexes 1-5 in DMSO are shown in Fig. 2 and the data are compiled in Table 1. These complexes display two absorption bands at 300 and 400 nm (Fig. 2). These bands can be assigned to a p-p* transition of the aromatic part and intraligand p-p* electronic transitions of the nonbonding electrons of the azomethine nitrogen atoms. Compared with 1,3 and 4 showed red-shifted absorption of 8 and 31 nm with decrease absorption intensity at 415 and 438 nm, respectively,indicating the strong electronic withdrawing effect of the substituent of diamines. Complexes 1-4 displayed weak and moderate fluorescence (lem 516,529,536,607 nm) with in DMSO when they are excited at 413,388,400,381 nm. Similar to absorption spectra,4 showed a significant red-shifted emission compared that of 1,which also reflects the electronic effect of diamine on 4. The fluorescence quantum yield of 0.17,0.10,0.06 and 0.04 were determined for 1-4,respectively,which indicated the emission capacities of Zntrisalen complexes decreased with the increasing of the electron-withdrawing abilities of the diamines. The fluorescence lifetimes of Zntrisalen complexes were also showed similar decreasing tendency with values obtained as 1.17, 0.67 and 0.55 for 1-3,respectively (the lifetime of 4 was too short to be determined in our instruments).

|
Download:
|
| Fig. 2.Normalized absorption and emission spectra in DMSO at 298 K of 1-5. | |
| Table 1 Photophysical properties of 1-5.a |
In non-coordinating solvents such as CH2Cl2,CHCl3 and n-hexane,the planar Znsalen complexes tend to aggregate through intermolecular Zn…O axial coordination [13]. This results in the structureless features in absorption spectra such as blue-shifts and larger bandwidths,compared to monomeric specie in coordinating solvents. To investigate the effect of cryptand structure on the existence of Znsalen,we compared the absorption spectra of 1 to that of 5 in CH2Cl2 in presence of different amount of DMSO. As shown in Fig. 3a,we found that 5 showed an envelope between 300 and 400 nm,a band at ca. 390 nm,and a shoulder centered at 430 nm. When 0-1500 eq. DMSO were titrated to the solution of 5 in DCM,a red-shifted structure between 300 and 400 nm is observed with the formation of a new,more intense band at ca. 405 nm. More importantly,two isosbestic points were present during the spectrophotometric titrations clearly indicate the transition from a dimmeric to monomeric species. Moreover, upon the addition of DMSO,the emission centered at 536 nm blue shifted to 508 nm,which a slight fluorescence decrease (14%). In addition,HR ESI-MS of 5 in DCM and DMSO solutions showed defined dimeric ([2M+H]+: 757.0760) and single species ([M+H]+: 379.0430),indicating complex 5 tends to aggregate in DCM (given in the Supporting information). From Fig. 3b,we found that DMSO titration does not lead significantly spectrophotometric and fluorimetric changes,which suggests that monomeric species in CH2Cl2 or DMSO solution lack of intermolecular Zn…O axial coordination.

|
Download:
|
| Fig. 3.Absorption and emission titration curve (λex= 390 nm for 5 and λex= 420 nm for 1) of 5 (a) and 1 (b) with addition of DMSO at 298 K. For 5,black line stand for the original state (0.05 mmol/L,2.5 x 10-8 mol in DCM) and red for the final state with 1500 eq. DMSO was added. For 1,black line stand for the original state (0.025 mmol/L, 1.25 x 10-8 mol in DCM) and red for the final state with 1000 eq. DMSO was added. (For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.) | |
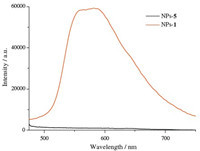
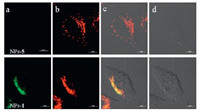
Since cryptand type Znsalen effectively reduces the selfaggregation of Znsalen which led to the quenched fluorescence in water to demonstrate the potential application of cryptand type Znsalen,we envisioned to encapsulate 1 or 5 in PLGA to demonstrate the remained fluorescence of 1 in HeLa cells,which would provide useful insights to construct Znsalen bioprobes. From Fig. 4,we found that,in the same concentration (1.0 mg/mL), NPs-1 exhibited higher fluorescence than NPs-5 (λex= 405 nm). As shown in Fig. 5,incubation with 0.10 mg/mL of NPs-1 for 12 h led to the green punctuate luminescence in a perinuclear pattern in HeLa cells (excited at 405 nm). Co-localization experiments revealed that the green fluorescence of NPs-1 overlapped well with the red fluorescence of the lysosome tracker and exhibited a co-localization level of ca. 60%,indicating NPs-1 mainly distributed in lysosomal organelles following internalization. However,NPs-5- treated HeLa cells showed almost invisible intracellular luminescence. These preliminary results demonstrate that the cryptand type structure efficiently reduces the self-aggregation of ZnSalen and remains the fluorescence in the interior of nanoparticles.
|
Download:
|
| Fig. 4.Emission spectra of NPs-5 and NPs-1 (λex = 405 nm). | |
|
Download:
|
| Fig. 5.Co-localization studies of the NPs with Lyso-Tracker Red: (a) images of the compounds indicated; (b) images of Lyso Tracker Red; (c) merged images of (a) and (b); (d) differential interference contrast (DIC). 0.10 mg/mL were incubated with HeLa cells for 12 h and then 50 nm Lyso-Tracker Red was incubated with HeLa cells for 20 min. Scale bar: 10 μm. | |
Taken together,we synthesized and characterized four cryptand type triZnsalen complexes and demonstrated their potential application in cell imaging experiments. Through comparative photophysical studies between 1 and 5 in noncoordination solvent CH2Cl2,we found that the robust and bulky cryptand structure could minimize intermolecular Zn…O interaction between Znsalen. Cell imaging results showed that 1 encapsulated by PLGA remains fluorescence intracellularly whereas 5 has no visible fluorescence in living cells. These results point to a new approach to prevent intermolecular Zn…O interaction and would be important to further designing luminescent Znsalen complexes as biological probes. Acknowledgment This project was supported by the National Scientific Foundation of China (No. 20971007) and National Key Basic Research Support Foundation of China (NKBRSFC) (Nos. 2013CB933402, 2015CB856300). Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.023.
| [1] | S.J. Wezenberg, A.W. Kleij, Material applications for salen frameworks, Angew. Chem. Int. Ed. 47 (2008) 2354-2364. |
| [2] | (a) T. Ueno, T. Koshiyama, M. Ohashi, et al., Coordinated design of cofactor and active site structures in development of new protein catalysts, J. Am. Chem. Soc. 127 (2005) 6556-6562; (b) P.F. Wang, Z.R. Hong, Z.Y. Xie, et al., A bis-salicylaldiminato Schiff base and its zinc complex as new highly fluorescent red dopants for high performance organic electroluminescence devices, Chem. Commun. (2003) 1664-1665; (c) L. Rigamonti, F. Demartin, A. Forni, S. Righetto, A. Pasini, Copper(II) complexes of salen analogues with two differently substituted (push-pull) salicylaldehyde moieties. a study on the modulation of electronic asymmetry and nonlinear optical properties, Inorg. Chem. 45 (2006) 10976-10989. |
| [3] | (a) S. Di Bella, I. Fragala, Two-dimensional characteristics of the second-order nonlinear optical response in dipolar donor-acceptor coordination complexes, New J. Chem. 26 (2002) 285-290; (b) J.L. Zhang, D.K. Garner, L. Liang, D.A. Barrios, Y. Lu, Noncovalent modulation of pH-dependent reactivity of a Mn-salen cofactor in myoglobin with hydrogen peroxide, Chem. Eur. J. 15 (2009) 7481-7489; (c) J.R. Carey, S.K. Ma, T.D. Pfister, et al., A site-selective dual anchoring strategy for artificial metalloprotein design, J. Am. Chem. Soc. 126 (2004) 10812-10813. |
| [4] | C.R. Bhattacharjee, G. Das, P. Mondal, S.K. Prasad, D.S.S. Rao, Novel green light emitting nondiscoid liquid crystalline zinc(II) Schiff-base complexes, Eur. J. Inorg. Chem. 2011 (2011) 1418-1424. |
| [5] | G.F. Qi, Z.Y. Yang, B.D. Wang, Synthesis, characterization and DNA-binding properties of zinc(II) and nickel(II) Schiff base complexes, Transit. Met. Chem. 32 (2007) 233-239. |
| [6] | (a) J. Jing, J.L. Zhang, Combining myeloperoxidase (MPO) with fluorogenic ZnSalen to detect lysosomal hydrogen peroxide in live cells, Chem. Sci. 4 (2013) 2947-2952; (b) J.J. Chen, J. Jing, H. Chang, et al., A sensitive and quantitative autolysosome probe for detecting autophagic activity in live and prestained fixed cells, Autophagy 9 (2013) 894-904. |
| [7] | Y. Hai, J.J. Chen, P. Zhao, et al., Luminescent zinc salen complexes as single and two-photon fluorescence subcellular imaging probes, Chem. Commun. 47 (2011) 2435-2437. |
| [8] | (a) J. Jing, J. Tang, D. Xie, et al., Design of luminescent ZnSalen for molecular imaging, Sci. Sin. Chim. 44 (2014) 191-203; (b) Y.B. Cai, J. Zhan, Y. Hai, J.L. Zhang, Molecular assembly directed by metal-aromatic interactions: control of the aggregation and photophysical properties of Zn-salen complexes by aromatic mercuration, Chem. Eur. J. 18 (2012) 4242-4249; (c) J. Tang, Y.B. Cai, J. Jing, J.L. Zhang, Unravelling the correlation betweenmetal induced aggregation and cellular uptake/subcellular localization of Znsalen: an overlooked rule for design of luminescent metal probes, Chem. Sci. 6 (2015) 2389-2397. |
| [9] | S.S. Sun, C.L. Stern, S.T. Nguyen, J.T. Hupp, Directed assembly of transition-metalcoordinated molecular loops and squares from salen-type components. Examples of metalation-controlled structural conversion, J. Am. Chem. Soc. 126 (2004) 6314-6326. |
| [10] | S. Akine, T. Taniguchi, T. Nabeshima, Helical metallohost-guest complexes via site-selective transmetalation of homotrinuclear complexes, J. Am. Chem. Soc. 128 (2006) 15765-15774. |
| [11] | O. Kotova, K. Lyssenko, A. Rogachev, et al., Low temperature X-ray diffraction analysis, electronic density distribution and photophysical properties of bidentate N,O-donor salicylaldehyde Schiff bases and zinc complexes in solid state, J. Photochem. Photobiol. A Chem. 218 (2011) 117-129. |
| [12] | G. Consiglio, S. Failla, P. Finocchiaro, I.P. Oliver, S.D. Bella, Aggregation properties of bis(salicylaldiminato)zinc(II) Schiff-base complexes and their Lewis acidic character, Dalton Trans. 41 (2012) 387-395. |
| [13] | G. Consiglio, S. Failla, P. Finocchiaro, et al., Supramolecular aggregation/deaggregation in amphiphilic dipolar Schiff-base zinc(II) complexes, Inorg. Chem. 49 (2010) 5134-5142. |
| [14] | S. Akine, S.J. Piao, M. Miyashita, T. Nabeshima, Cage-like tris(salen)-type metallocryptand for cooperative guest recognition, Tetrahedron Lett. 54 (2013) 6541-6544. |
| [15] | C.A. Strassert, L.E. Dicelio, J. Awruch, Reduction of an amido zinc(II) phthalocyanine by diborane, Synthesis (2006) 799-802. |
| [16] | A. Heaton, M. Hill, F. Drakesmith, Polyhalogenonitrobenzenes and derived compounds Part 5. Improved preparations of 1,2,3,4-tetrafluoro-5,6-dinitrobenzene and 3,4,5,6-tetrafluoro-1,2-phenylenediamine, and the use of the latter for the synthesis of tetrafluorobenzheterocycles, J. Fluor. Chem. 81 (1997) 133-138. |
| [17] | K. Li, J. Pan, S.S. Feng, et al., Generic strategy of preparing fluorescent conjugatedpolymer-loaded poly(DL-lactide-co-glycolide) nanoparticles for targeted cell imaging, Adv. Funct. Mater. 19 (2009) 3535-3542. |
| [18] | D.N. Kumar, B.S. Garg, Synthesis and spectroscopic studies of complexes of zinc(II) with N2O2 donor groups, Spectrochim. Acta A Mol. Biomol. Spectrosc. 64 (2006) 141-147. |