b Wuxi Medical School, Jiangnan University, Wuxi 214122, China;
c Synergetic Innovation Center of Food Safety and Nutrition, Jiangnan University, Wuxi 214122, China
As demand for discovering new molecules is increasing day by day,the requirements are not adequately met by the traditional methods,which have prompted chemists to work on alternative concepts to create new molecules at much faster rate as they can [1]. Both carbohydrates and amino acids are widely used in nature as fundamental building blocks to build its vast repertoire of biomolecules,which can be amalgamated to create nature-like and yet unnatural new molecules with multifunctional groups anchored on a single of ensemble [2]. Sugar amino acids (SAAs), the carbohydrate derivatives bearing both amino and carboxylic acid functional groups,represent an important class of such new molecules that can be used to create novel materials with potential applications as glycomimetics,artificial amino acids and peptidomimetics [3].
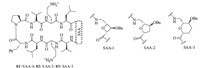
In this review,we describe recent synthetic strategies of SAAs as a novel class of building blocks and their applications in creating large number of structurally diverse glycomimetics and peptidomimetics. The term SAAs was used for compounds that are basically hybrids of carbohydrate and amino acids where carboxyl and amino functional groups have been incorporated at the two termini of regular 2,5- or 2,6-anhydrocarbohydrate frameworks (Fig. 1).
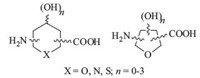
|
Download:
|
| Fig. 1.General structures of SAAs. | |
SAAs and their derivatives occur in nature in various forms (Fig. 2). The most prominent example is sialic acid (1),a family of N- or O-substituted derivatives of neuraminic acid,containing over 50 derivatives of the nine-carbon backbone. These structures unusually appear free in nature,but present as components of oligosaccharide chains of mucins,glycoproteins and glycolipids occupying terminal,non-reducing positions of complex carbohydrates on both external and internal membrane areas where they are very exposed and develop important functions [4]. Glucosaminuronic acid (2),which has many biological functions such as being a component of many typical bacterial cell walls,exists naturally as an acetyl derivative [5]. The naturally occurring 2-acetamido-2-deoxygalacturonic acid (3) is one of bacterial Vi-antigen components of Escherichia coli [5]. 2-Amino-2-deoxy- D-mannouronic acid (4) is found in bacterial polysaccharide sequences. Derivatives of 3-amino-3-deoxy-D-gulopyranuronic acid (5) and 3-amino-3,4-dideoxy-D-xylohexopyranuronic acid (6) are found in ezomycin A [5]. Naturally existing 4-amino-4- deoxy-glucuronic acid (7) is found in gougerotin,a product isolated from Streptomyces bacteria [6]. SAA 8 is found as the residue of the cell wall O-antigen in Plesiomonas shigelloides O51 [7]. Peptidyl nucleoside antibiotics polyoxin (9),nikkomycin (10) are other kinds of natural SAAs [8]. Hydroxylated prolines (11) and hydroxylated pipecolic acids (12) are azasugar-based SAAs,whose endocyclic oxygen atom in the ring is replaced with a nitrogen atom [9]. The naturally occurring SAA 13 is the a,a-disubstituted hydantoin derivative,exhibiting potent selective anti-herbal activity with no toxicity to microorganisms or animals [10].
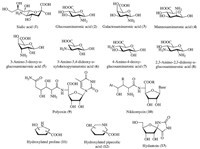
|
Download:
|
| Fig. 2.Examples of naturally occurring SAAs. | |
In recent years,a number of research groups have designed and synthesized many unnatural SAAs and used them to create novel structural entities. The synthesis of SAAs is always accomplished in a few steps starting from commercially available or easily accessible monosaccharides,such as glucose,galactose,glucosamine, diacetone glucose ect. In order to obtain synthetic SAAs, amino and carboxylic functional groups are required to be introduced. The amino functional group can be introduced as azide,cyanide,nitromethane,followed by subsequent reduction. The carboxylic group can be constructed by selective oxidation of a primary alcohol,or by Wittig reaction and subsequent oxidation,or directly as carbon dioxide,or as a hydrolyzable cyanide and subsequent hydrolysis reaction.
3.1. Introduction of amino group 3.1.1. Introduction of azide
As shown in Scheme 1,addition of hydrazoic acid to the a,b-unsaturated aldehyde derived from methyl 3,4-di-O-acetyl-Dglucuronal (14),followed by glycosylation of methanol,yielded a mixture of products that were separated and identified as methyl (methyl 4-O-acetyl-3-azido-2,3-dideoxyhexopyranosid)uronates (-a-D-arabino (15a),-b-D-ribo (15b),-a-D-ribo- (15c),and -b-Darabino (15d)) [11]. Compounds 15a-d were each O-deacetylated with sodium methoxide in methanol (0.1 mol/L) to yield the corresponding methyl (methyl 3-azido-2,3-dideoxyhexopyranosid) uronates,which were then reduced by hydrogenation (10% Pd/C) of the azide to yield methyl (methyl 3-amino-2,3-dideoxy-a-Darabino- (16a),-b-D-ribo- (16b),-a-D-ribo- (16c) -b-D-arabinohexopyranosid) uronates (16d). Treatment of 16a and 16d with 1 mol/L aq. NaOH led to completely unprotected SAAs 17a and 17b [12].

|
Download:
|
| Scheme. 1.Synthesis of SAAs 17a-b. | |
The substitution of an anomeric O-acyl group with cyanide of trimethylsilyl cyanide (TMSCN) in the presence of a mild catalyst was reported by Gross P.H. [13]. As shown in Scheme 2,treatment with excess TMSCN and 0.1 mol equiv. of HgBr2 in CH3NO2,b-Dglucopyranose 18 was converted into b-D-glucosyl cyanide 19 in good yield. Then reduced 19 with LiAlH4 in THF solution, protected of the resulting amine with FmocCl in 10% Na2CO3 solution,and oxidated by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO),NaOCl,Bu4NCl,NaBr in CH2Cl2 solution they obtained SAA 20 [14].

|
Download:
|
| Scheme. 2.Synthesis of SAA 20. | |
The synthesis of b-D-galactopyranosyl-N-(fluoren-9-ylmethoxycarbonyl) methane 22 was reported in Scheme 3 [15]. CH3NO2 was introduced to b-D-galactose via nucleophilic aldol reaction to yield the nitro compound 21. Reduction of 21 with H2 using 10% Pd/C, protection of the resulting amine with FmocCl,and selectively oxidation by TEMPO catalyzed sodium hypochlorite afforded 22 in 74% yield for the three-step sequence.

|
Download:
|
| Scheme. 3.Synthesis of Fmoc-SAA-OH 22. | |
The synthesis of SAA 24 was described in Scheme 4. Mannuronic acid 23 was treated with NH4HCO3 in water to form SAA 24 [16]. This method is useful for the amination of the anomeric hydroxyl group. Yet it is rarely used in the synthesis of glycoconjugates.
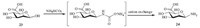
|
Download:
|
| Scheme. 4.Synthesis of Fmoc-SAA-OH 24. | |
Selective oxidation of a primary alcohol was shown in Scheme 5 [17]. Treatment of glycosyl azide 25 [18] with NaOMe in methanol, followed by hydrogenation with H2 and Pd/C in methanol,and amino protective reaction using fluoren-9-ylmethoxycarbonyl-Osuccinimide (Fmoc-OSu) in pyridine provided the Fmoc-protected amine 26. The C-6 hydroxyl of 26 was then selectively oxidized by TEMPO-catalyzed NaOCl oxidation with careful pH control to generate N-(fluoren-9-ylmethoxycarbonyl)-b-D-glucopyranosylamine uronic acid 27.

|
Download:
|
| Scheme. 5.Synthesis of Fmoc-SAA 27. | |
Synthesis of Boc-protected furanoid SAA 32 using Wittig reaction was shown in Scheme 6. 2,3-O-isopropylidene-protected ribose 28 was reacted with methyl(triphenylphosphoranylidene) acetate by a Wittig reaction to afford b-C-furanoside 29 in 86% yield [19]. Treatment 29 with methanesulfonyl chloride (MsCl) in pyridine,subsequently substitution the intermediate mesylate with NaN3 afforded compound 30 in a yield of 87%. Palladiumcatalysed hydrogenation of 30 in the presence of an equimolar amount of hydrochloric acid yielded the amine as its hydrochloric salt,which was then treated with Boc2O under Schotten-Baumann condition to obtain carbamate 31 in 82% yield. Hydrolysis of 31 with NaOH yielded the desired SAA 32 in 89% yield [20].
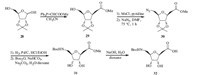
|
Download:
|
| Scheme. 6.Synthesis of Boc-SAA 32. | |
Synthesis of protected building block SAA 38 (Scheme 7) was achieved by directly introduced the carboxyl with CO2 [21]. The amino function of 33 was protected by benzyl carbonochloridate (CbzCl) to obtain 34 in 90% yield. Chlorination of the anomeric hydroxyl group provided the a-chloro compound which was treated with tributyltin lithium at -78 ° to afford 35 in 79% yield. The generation of the glycosyl dianion 36 was accomplished in two separate temperature steps: firstly,deprotonation of the urethane nitrogen at -78 ° using 1 equiv. of BuLi; secondly,transmetalation at -55 ° using 1.2 equiv. of BuLi. The dianion 36 was visualized as a deep red color in solution and was subsequently trapped by CO2 to afford 37 in 83% yield. Cleavage the Cbz-group of 37 by using 2.5 equiv. of trimethylsilyl iodide (TMS-I) in CH3CN, and then treated with N-(9-fluorenylmethoxycarbonyloxy)succinimide (Fmoc-ONSu) to afford 38 in 48% yield.

|
Download:
|
| Scheme. 7.Synthesis of the protected building block SAA 38. | |
Synthesis of SAA 44 was described in Scheme 8 [22]. Employed D-glucosamine hydrochloride as the starting material,the 1-cyano- 2-phthalimido derivative 39 was gained in four steps according to the published procedure [23]. Treatment of 39 with 30% HBr-AcOH at 0 ° to room temperature generated the methanamide derivative 40 in 85% yield,which was subsequently treated with Dowex 50WX8 [H+] in refluxing MeOH to give the methyl ester derivative 41 in 97% yield. Removal of the phthaloyl group was accomplished by the successive treatment of 41 with aqueous LiOH and 3 mol/L HCl to give an amine derivative 42 as a hydrochloride salt in 95% overall yield,protection of the amino group of 42 with BOC-ON,followed by esterification of the C-1 carboxylate with 2-bromoacetophenone and conventional acetylation of hydroxyl groups gave 43 in 73% overall yield. Finally,hydrogenation of43 withH2 and Pd/C afforded 44 in 90% yield.

|
Download:
|
| Scheme. 8.Synthesis of the protected building block SAA 44. | |
SAAs are broadly defined as carbohydrate-derived structures bearing both carboxylic acid and amino functional groups,thus SAAs can be viewed as carbohydrate mimics or as amino acid mimics. Carbohydrates present in glycopeptides,glycolipids and nucleotides play very important roles in various biological processes,especially in cell-cell recognition [24]. While amino acids represent an important class of building blocks used for the synthesis of polyamide,peptides,and proteins [25]. In recent years, SAAs have emerged as versatile and multifunctional building blocks used extensively in many studies,especially in glycomimetics and peptidomimetics.
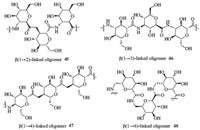
4.1. Glycomimetics 4.1.1. Linear oligomersFour types of homo-oligomers,b(1!2)-linked oligomer 45, b(1!3)-linked oligomer 46,b(1!4)-linked oligomer 47,and b(1!6)-linked oligomer 48 (Fig. 3) were synthesized in Ichikawa’s group [26]. It was determined that only the b(1!2)-linked oligomer 45 possessed a helical structure that seemed to be predetermined by the linkage position through circular dichroism (CD) and nuclear magnetic resonance (NMR) spectral studies. Homo-oligomers with b(1!2)-linkages 45 and b(1!6)-linkages 48 were also subjected to O-sulphation,and these O-sulphated oligomers were found to be able to effectively inhibit L-selectin-mediated cell adhesion,HIV infection,and heparanase activity without the anticoagulant activity associated with naturally occurring sulfated polysaccharides such as heparin.
|
Download:
|
| Fig. 3.Four types of linear oligomers 45-48. | |
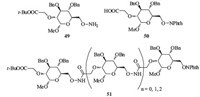
The novel 2,6-linked oligosaccharide mimetics derived from glycoaminoxy acid building unit was reported recently (Fig. 4) [27]. Firstly,2,6-functionalized pyranoid glycoaminoxy acids (49 and 50) were efficiently synthesized in which aminoxy and carboxylic acid functions had been easily introduced at 2- and 6-position of a commercially available methyl 3,4-di-O-benzyla- D-glucopyranoside template. Subsequently,assembly of this glycoaminoxy acid building unit via N-acylation led successfully to the novel 2,6-linked oligosaccharide mimetics (51).
|
Download:
|
| Fig. 4.Synthesis of 2,6-linked oligosaccharide mimetics 51. | |
The first generation of branched SAA oligomers synthesized in solution phase via two main routes: By use of a standard coupling reagent and via use of active ester intermediates. As shown in Fig. 5,from the branched d-3,5-trans-tetrahydrofuran (THF) SAA 52a,it was possible to obtain a benzylprotected dimeric carbopeptoid and methyl-protected dimeric and tetrameric, hexameric and octameric carbopeptoids (53). While from the branched d-3,5-cis-THF SAA 52b,it was possible to achieve the synthesis of methyl-protected dimeric and tetrameric carbopeptoids (54). The author found these oligomers have potential foldameric properties. Amongst many uses,foldamers provide simpler models in the study of the factors which induce folding and unfolding of proteins and,ultimately,potential insights into their functioning [28].

|
Download:
|
| Fig. 5.First generation of branched SAA oligomers 53 and 54 synthesized from two branched d-3,5-THF building blocks 52a-b. | |
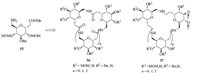
Xie’s group reported a new family of orthogonally protected cyclic homooligomers with two to four sugar units derived from pyranoid SAAs [29]. As shown in Fig. 6,cyclic oligomers composed of amide-linked SAAs (56) were prepared by cyclization of linear oligomers of the novel orthogonally protected pyranoid SAA 55 using a solution-phase coupling method. These orthogonally protected cyclic molecules can be selectively or fully de-protected, to afford the macrocycles ready to further functionalization. These macrocycles can be used as host molecules in both organic and aqueous environment. Furthermore,reduction of amide bonds in cyclic oligomers 56 generated the corresponding amine-linked macrocycles 57,which represent the first example of this type of carbohydrate-derived host molecules. In all cyclic compounds,deprotected trimers and tetramers displayed a 4C1 chair conformation with oxygen atoms of the sugar ring located on the interior of the cavity and the secondary hydroxyl groups outward.
|
Download:
|
| Fig. 6.Structures of cyclic oligomers 56 and 57. | |
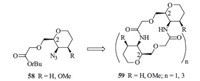
Recently,Martı´n T. et al. reported the synthesis of cyclic homooligomers from pyranoid e-SAAs analogs (Fig. 7) [30]. Firstly, pyranoid e-SAAs analogs (58) were designed and synthesized as building blocks,in which the carboxylic acid and the amine groups were installed in positions C2 and C3 with respect to the tetrahydropyran oxygen atom. This disposition allowed such an oxygen atom to participate actively in the control of the intramolecular hydrogen-bonding pattern,and therefore to affect the conformation equilibria. Secondly,cyclic oligomers (59) containing pyranoid e-SAAs building blocks were synthesized by using standard solution-phase coupling procedures. Thirdly,conformation analysis of 59 was performed using NMR,X-ray,FTIR,and a theoretical conformation search. More importantly,it revealed that the presence of methoxy group in the position C4 of the pyran ring produced an important structural change in the cyclic oligomers. When the methoxy group was absent,a U-shaped structure was adopted,in which a hydrophilic concave face with four oxygen atoms and two amide protons directed toward the center of the cavity were found. However,with the presence of methoxy groups,the structure collapsed through inter-residue hydrogen bonds between the oxygen atoms of the pyran ring and the amide protons.
|
Download:
|
| Fig. 7.Structures of the cyclic oligomers 59 designed based on e-SAA units 58. | |
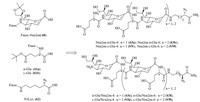
As shown in Fig. 8,Gervay-Hague et al. [31] reported a microwave-assisted solid-phase peptide syntheses and structural characterization studies of a series of water-soluble a/d hybrid peptides (63a-h) derived from Fmoc-Neu2en (60),Fmoc- Glu(OtBu)-OH (61) and Fmoc-a-azido-e-aminocaproic acid linker (N3Lys,62). Secondary structures of these hybrid foldamers were studied through conformational experiments,including CD,NH/ ND exchange,and ROESY in aqueous solution. It was founded that 63a-f were mismatched and were random coiled in aqueous solution,while 63g-h could be matched and formed stable secondary structures. This study provided a fundamental understanding of the factors that influence stable secondary structure in hybrid Neu2en/Glu systems,and the tools to establish a viable platform for the rational design of a-(2,8)-polysialic acid surrogates.
|
Download:
|
| Fig. 8.Structures of a/d hybrid peptides 63a-h. | |
Mark Overhand’s group [32] synthesized and evaluated six epoxomicin-derived SAA containing peptide epoxyketones 65 (Fig. 9). Firstly, 65 were designed and synthesized from SAA scaffolds (64) using solution-phase peptide synthesis protocols (Fig. 9). Secondly,a proteasome inhibitor competition assay was made for the evaluation of 65. It revealed that none of these compounds displayed inhibitory activity toward any of the proteasome active sites,but their approach hold promise toward the development of structurally new proteasome inhibitors.

|
Download:
|
| Fig. 9.Structures of linear peptidomimetics 65 derived from 64. | |
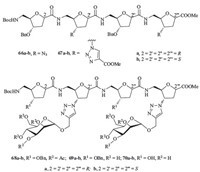
Chakraborty T.K. and co-workers [33] described the effects of appended sugar moieties on the conformational behavior of glycopeptide mimics (66-70) derived from d-SAA (Fig. 10). The glycopeptide mimics 66-70 were differed from one another in the configuration of the stereocenter at C2 of the furanoid rings of their constituent d-SAA moieties (those with the ‘‘2S’’ configuration designated cis-foldamers,and those with ‘‘2R’’,trans-foldamers). It was particularly significant that the interactions of the cis- and trans-glycofoldamers 70a and 70b with both lectin ConA and bacterium E. coli were distinct,suggesting that these differences may have their seeds in the underlying conformational preferences of this pair of neoglycopeptides.
|
Download:
|
| Fig. 10.Structures of the glycopeptide mimics 66-70. | |
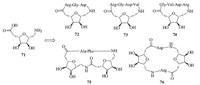
Overhand M. and co-workers [34] used furanoid e-SAA 71 to assemble some cyclic Arg-Gly-Asp (RGD) peptides 72-76 (Fig. 11) by solid-phase method using a cyclization-cleavage protocol. NMR-based molecular dynamics simulations and empirical calculations of the cyclic tetramer 75 showed that it was conformationally restrained with two SAA units adopting different conformations. Subsequently,ability of cyclic RGD peptides 72-76 to bind to the integrin receptors avb3 and aIIbb3 were investigated. Cyclic tetrapeptide 72 showed the most promising activity in an inhibition assay with an IC50 of 1.49 mmol/L for the avb3 receptor and 384 nmol/L for the aIIbb3 receptor.
|
Download:
|
| Fig. 11.Cyclic peptides 72-76 containing furanoid e-SAA 71. | |
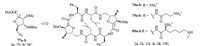
A set of novel SAA based cationic antimicrobial peptides (CAPs) were designed and synthesized by Kunwar A.C. et al. [35]. They used the functionally tuned SAA building blocks 77a-b to synthesize 24-membered macrocyclic C2-symmetric cationic peptides 78-80 (Fig. 12). The conformation of these cyclic peptides was founded to resemble the dumbbell-shaped loloatin cyclopeptides more than the typical b-sheet structures of gramicidin S or tachyplesins through NMR techniques. Fortunately,all cyclic CAPs, with exception of 78b,showed pronounced antimicrobial activity against for Gram-negative (E. coli and Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus and Bacillus subtilis) bacteria, and low hemolytic activity. They believed that the encouraging biological activities of these compounds may be useful to generate new CAP-based drugs.
|
Download:
|
| Fig. 12.Structures of cyclic CAPs 78-80 containing SAA 77. | |
Mark Overhand’s group focused on the study of gramicidin S (GS) analogs featuring SAA dipeptide isosteres in one or both turn regions. As shown in Fig. 13,they evaluated the effect of ring size of SAAs as type II0 b-turn mimics on the structural and biological properties of GS-derived cyclic peptides [36]. Homologues 81-83 (containing a monobenzylated oxetane,furanoid,and pyranoid SAA,respectively) exhibit well-defined cyclic hairpin structures in solution. By using CD,NMR spectroscopy,modeling,and X-ray diffraction,they found that the ring size of the incorporated SAA moieties determined the spatial positioning of their cis-oriented carboxyl and aminomethyl substituents,thereby subtly influenced the amide linkages with the adjacent amino acids in the sequence. They identified that 83 was slightly less hydrophobic than 81 and 82,and displays a diminished haemolytic activity. Thus,they considered that 81-83 can be used for the future design of compounds with enhanced biological properties remaining subject for further research.
|
Download:
|
| Fig. 13.Structures of GS-derived cyclic peptides 81-83. | |
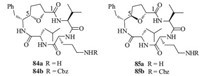
Chakraborty’s group [37] recently reported conformational analysis of four 15-membered cyclic tetra-peptides (CTPs,84-85) with a3d architecture containing SAA (Fig. 14). Conformational analyses of four CTPs,carried out in DMSO-d6 using various NMR techniques,supported by restrained MD calculations. A rare phenomenon was demonstrated that fused bg-turn structures in a cyclic tetrapeptide with a3d architecture. The a3d macrocycles got stabilized by both 10-membered b-turn as well as a 7-membered g-turn,fusing within the same macrocycle. Moreover,the stereocenter variation at C5 did not affect the fused turn structures and exhibited similar conformations in both protected forms (84b and 85b) and de-protected forms (84a and 85a),This discovery was highly advantageous as fused reverse turn structures present in the cyclic structure with minimalistic size macrocycle,which could be applied to develop suitable pharmacophores in the drug development process.
|
Download:
|
| Fig. 14.Structures of cyclic tetrapeptides 84 and 85 containing SAA. | |
The synthetic strategies and applications of SAAs have been reviewed,as they represent unique structures among carbohydrates and peptides. The synthetic strategies of SAAs include two aspects,construction of amino and carboxylic functional groups. Amino functional groups could be installed by introduction of azide,cyanide,nitromethane and ammonium bicarbonate. Construction of carboxylic function group could be achieved by selective oxidation of a primary alcohol,Wittig reaction,directly introduced as carbon dioxide or as a hydrolyzable cyanide and subsequent hydrolysis reaction.
SAAs are fast emerging as an important class of multifunctional building blocks that can find wide-ranging applications. SAAs have been used to generate simple glycomimetic libraries through derivatization and oligomerization of the amino acid moiety. Biological evaluation of these glycomimetics could discover potent inhibitors of carbohydrate-protein binding. Besides being sugarlike, SAAs are also used in peptidomimetics as rigid templates capable of inducing secondary structures in peptides. The various functional groups of SAAs can serve as adapters for solid-phase synthetic methods providing opportunities to create libraries of multifaceted molecules that will lead to the development of more and more bioactive molecules.
The synthesis and biological evaluation of SAA building blocks in glycomimetics and peptidomimetics systems keep expanding the reach of the glycosciences to the drug discovery and medical sciences. It provides a great outlook on the wide range of cellular functions of carbohydrates and their derivatives involved,as well as good insight into the nature of oligosaccharide and protein folding.
AcknowledgmentsThis studywas supported by the National Science Foundation for Young Scientists of China (No. 21302068),the Natural Science Foundation of Jiangsu Province,China (No. BK20130127),Jiangsu Province ‘‘Six Summit Talent’’ Foundation (No. 2012-SWYY-009),the Specialized Research Fund for the Doctoral Program of Higher Education (No. 20120093120002) and the Fundamental Research Funds for theCentral Universities (Nos. JUSRP51319B,JUSRP51411B).
| [1] | T.K. Chakraborty, P. Srinivasu, S. Tapadar, B.K. Mohan, Sugar amino acids and related molecules: some recent developments, J. Chem. Sci. 116 (2004) 187-207. |
| [2] | T.K. Chakraborty, P. Srinivasu, S. Tapadar, B.K. Mohan, Sugar amino acids in designing new molecules, Glycoconj. J. 22 (2005) 83-93. |
| [3] | S.A.W. Gruner, E. Locardi, E. Lohof, H. Kessler, Carbohydrate-based mimetics in drug design: sugar amino acids and carbohydrate scaffolds, Chem. Rev. 102 (2002) 491-514. |
| [4] | X. Chen, A. Varki, Advances in the biology and chemistry of sialic acids, ACS Chem. Biol. 5 (2010) 163-176. |
| [5] | J. Gervay-Hague, J.T.M. Weathers, Pyranosyl sugar amino acid conjugates: their biological origins, synthetic preparations, and structural characterization, J. Carbohydr. Chem. 21 (2002) 867-910. |
| [6] | M.T. Migawa, L.M. Risen, R.H. Griffey, E.E. Swayze, An efficient synthesis of gougerotin and related analogues using solid-and solution-phase methodology, Org. Lett. 7 (2005) 3429-3432. |
| [7] | A. Maciejewska, J. Lukasiewicz, T. Niedziela, Z. Szewczukc, C. Lugowski, Structural analysis of the O-specific polysaccharide isolated from Plesiomonas shigelloides O51 lipopolysaccharide, Carbohydr. Res. 344 (2009) 894-900. |
| [8] | G.Q. Niu, H.R. Tan, Nucleoside antibiotics: biosynthesis, regulation, and biotechnology, Trends Microbiol. 23 (2015) 110-119. |
| [9] | M. Risseeuw, M. Overhand, G.W.J. Fleet, M.I. Simone, A compendium of cyclic sugar amino acids and their carbocyclic and heterocyclic nitrogen analogues, Amino Acids 45 (2013) 613-689. |
| [10] | M. Nakajima, K. Itoi, Y. Takamatsu, et al., Hydantocidin: a new compound with herbicidal activity from Streptomyces hygroscopicus, J. Antibiot. (Tokyo) 44 (1991) 293-300. |
| [11] | D. Tuwalska, A. Sikorski, B. Liberek, Synthesis and geometry of methyl (methyl 4-O-acetyl-3-azido-2,3-dideoxy-α/β-D-arabino-and -α/β-D-ribo-hexopyranosid) urinates, Carbohydr. Res. 343 (2008) 404-411. |
| [12] | D. Tuwalska, J. Sienkiewicz, B. Liberek, Synthesis and conformational analysis of methyl 3-amino-2, 3-dideoxyhexopyranosiduronic acids, new sugar amino acids, and their diglycotides, Carbohydr. Res. 343 (2008) 1142-1152. |
| [13] | P. Phiasivongsa, J. Gallagher, C.N. Chen, et al., Palladium-charcoal-catalyzed reduction of tri-O-acetyl-β-L-fucopyranosyl cyanide: a route to small cluster oligosaccharide mimetics (SCOMs), Org. Lett. 4 (2002) 4587-4590. |
| [14] | E. Lohof, E. Planker, C.Mang, et al., Carbohydrate derivatives for use in drug design: cyclicαv-selective RGD peptides, Angew. Chem. Int. Ed. Engl. 39 (2000) 2761-2764. |
| [15] | A.E.J. de Nooy, A.C. Besemer, et al., Selective oxidation of primary alcohols mediated by nitroxyl radical in aqueous solution. Kinetics and mechanism, Tetrahedron 51 (1995) 8023-8032. |
| [16] | E. Kallin, Use of glycosylamines in preparation of oligosaccharide polyacrylamide copolymers, Methods Enzymol. 242 (1994) 221-226. |
| [17] | L.Q. Ying, J. Gervay-Hague, Synthesis of N-(fluoren-9-ylmethoxycarbonyl)glycopyranosylamine uronic acids, Carbohydr. Res. 339 (2004) 367-375. |
| [18] | L.Q. Ying, J. Gervay-Hague, General methods for the synthesis of glycopyranosyluronic acid azides, Carbohydr. Res. 338 (2003) 835-841. |
| [19] | J.P. McDevitt, P.T. Lansbury Jr., Glycosamino acids: new building blocks for combinatorial synthesis, J. Am. Chem. Soc. 118 (1996) 3818-3828. |
| [20] | (a) R.M. van Well, H.S. Overkleeft, M. Overhand, et al., Parallel synthesis of cyclic sugar amino acid/amino acid hybrid molecules, Tetrahedron Lett. 41 (2000) 9331-9335; (b) R.M. van Well, L. Marinelli, K. Erkelens, et al., Synthesis and structural analysis of cyclic oligomers consisting of furanoid and pyranoid e-sugar amino acids, Eur. J. Org. Chem. 12 (2003) 2303-2313. |
| [21] | E.G. von Roedern, E. Lohof, G. Hessler, M. Hoffmann, H. Kessler, Synthesis and conformational analysis of linear and cyclic peptides containing sugar amino acids, J. Am. Chem. Soc. 118 (1996) 10156-10167. |
| [22] | Y. Suhara, M. Kurihara, A. Kittaka, Y. Ichikawa, Efficient synthesis of carbopeptoid oligomers: insight into mimicry of b-peptide, Tetrahedron 62 (2006) 8207-8217. |
| [23] | R.W. Myers, L.C. Lee, Synthesis and characterization of some anomeric pairs of per-O-acetylated aldohexopyranosyl cyanides (per-O-acetylated 2,6-anhydroheptononitriles). On the reaction of per-O-acetylaldohexopyranosyl bromides with mercuric cyanide in nitromethane, Carbohydr. Res. 132 (1984) 61-82. |
| [24] | T.K. Chakraborty, S. Ghosh, S. Jayaprakash, Sugar amino acids and their uses in designing bioactive molecules, Curr. Med. Chem. 9 (2002) 421-435. |
| [25] | F. Schweizer, Unusual amino acids accessed through sugar-amino acid hybrids and incorporation into biologically active peptides, Trends Glycosci. Glycotechnol. 15 (2003) 315-328. |
| [26] | Y. Suhara, Y. Yamaguchi, B. Collins, et al., Oligomers of glycamino acid, Bioorg. Med. Chem. 10 (2002) 1999-2013. |
| [27] | Z. Song, X.P. He, G.R. Chen, J. Xie, 6-O-amino-2-O-carboxymethyl glucopyranoside as novel glycoaminoxy acid building block for the construction of oligosaccharide mimetics, Synthesis 17 (2011) 2761-2766. |
| [28] | M.I. Simone, A.A. Edwards, G.E. Tranter, G.W.J. Fleet, C-3 branched d-3, 5-cis-and trans-THF sugar amino acids: synthesis of the first generation of branched homooligomers, Amino Acids 41 (2011) 643-661. |
| [29] | M. Mé nand, J.C. Blais, L. Hamon, J.M. Valé ry, J. Xie, Synthesis of orthogonally protected cyclic homooligomers from sugar amino acids, J. Org. Chem. 70 (2005) 4423-4430. |
| [30] | A. Feher-Voelger, J. Borges-Gonzá lez, R. Carrillo Dr, et al., Synthesis and conformational analysis of cyclic homooligomers from pyranoid e-sugar amino acids, Chem. Eur. J. 20 (2014) 4007-4022. |
| [31] | J.P. Saludes, J.B. Ames, J. Gervay-Hague, Synthesis and structural characterization of sialic acid-glutamic acid hybrid foldamers as conformational surrogates of a-2, 8-linked polysialic acid, J. Am. Chem. Soc. 131 (2009) 5495-5505. |
| [32] | M.D.P. Risseeuw, B.I. Florea, G.A. van der Marel, H.S. Overkleeft, M. Overhand, Sugar amino acid based peptide epoxyketones as potential proteasome inhibitors, Bioorg. Chem. 38 (2010) 202-209. |
| [33] | A. Siriwardena, K.K. Pulukuri, P.S. Kandiyal, et al., Sugar-modified foldamers as conformationally defined and biologically distinct glycopeptide mimics, Angew. Chem. Int. Ed. 52 (2013) 10221-10226. |
| [34] | (a) R.M. van Well, L. Marinelli, C. Altona, et al., Conformational analysis of furanoid ε-sugar amino acid containing cyclic peptides by NMR spectroscopy, molecular dynamics simulation, and X-ray crystallography: evidence for a novel turn structure, J. Am. Chem. Soc. 125 (2003) 10822-10829; (b) R.M. van Well, H.S. Overkleeft, G.A. van der Marel, et al., Solid-phase synthesis of cyclic RGD-furanoid sugar amino acid peptides as integrin inhibitors, Bioorg. Med. Chem. Lett. 13 (2003) 331-334. |
| [35] | T.K. Chakraborty, D. Koley, R. Ravi, et al., Synthesis, conformational analysis and biological studies of cyclic cationic antimicrobial peptides containing sugar amino acids, J. Org. Chem. 73 (2008) 8731-8744. |
| [36] | A.D. Knijnenburg, A.W. Tuin, E. Spalburg, et al., Exploring the conformational and biological versatility of β-turn-modified gramicidin S by using sugar amino acid homologues that vary in ring size, Chem. Eur. J. 17 (2011) 3995-4004. |
| [37] | S. Gajendra, G. Uttam, P. Sudip, et al., βγ-fused turn structures in sugar amino acid (SAA) containing cyclic tetrapeptides with a3d architecture, Tetrahedron 70 (2014) 7681-7685. |




