b Shanghai Engineering Research Center of Molecular Therapeutics and New Drug Development, East China Normal University, Shanghai 200062, China;
c Medicinal Chemistry, ChemBridge Research Laboratories Inc., San Diego, CA 92127, USA;
d School of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China
Calix[n]arenes [1] are macrocyclic compounds with interesting conformational and cavity structures,and the easy functionalization of the rims (both upper and lower rims) of calix[n]arene skeletons makes them ideal candidates for many potential applications in supramolecular chemistry,such as host-guest chemistry [2],molecular encapsulation [3],and as scaffolds for the construction of multivalent ligands [4]. Calix[4]arene,the smallest member of the calix[n]arene family,can possess four different conformations and each of the four conformational structures can be geometrically locked. Thus,the spatial arrangement of the functional groups attached on the rims of calix[4]arenes could be controlled. The introduction of new functional groups into the calix[n]arene skeleton would certainly diversify its structural and functional properties and broaden its applications in the area of supramolecular chemistry.
Malonate is a naturally occurring substance possessing two carboxylate functions,and was used as a competitive inhibitor of the enzyme succinate dehydrogenase [5]. We envisioned that introduction of malonate derivatives to the calix[4]arene backbone would afford new calix[4]arene structures with malonate derivative-based interactive sites,and these malonate derivatives might be chemically transformed to variety of other functional groups, such as amino,amide,carboxyl and hydroxyl groups. These functional groups would broaden the application of calix[4]arene in the areas of molecular recognition and host-guest chemistry,or even find their practical use in biological and environmental fields [5, 6]. However,research work toward these goals was almost absent in literature report [7]. Herein,we report the synthesis and structure characterization of six calix[4]arenes with their upper rims being functionalized by different number of malonate derivatives. 2. Experimental
Commercially available chemicals were used without further purification unless stated otherwise; compounds 1 and 2 were synthesized according to literatures [8, 9, 10]. 1H NMR and 13C NMR spectra were recorded on a Varian Mercury 500 spectrometer in CDCl3 with TMS as the reference. Mass spectra were recorded on a microTOF QII mass spectrometer (Bruker Daltonics,Germany). Single crystal X-ray diffraction data were collected on a Bruker SMART APEX 2 X-ray diffractometer equipped with a normal focus Mo-target X-ray tube ( λ = 0.71073 Å ) and data reduction included absorption corrections by the multi-scan method. The structures were solved by direct methods and refined by full-matrix leastsquares using SHELXS-97. 2.1. Synthesis of symmetric substituted calix[4]arenes 3a- c
To a round bottom flask containing a methanol solution (20 mL) of tetra-formyltetrapropoxycalix[4]arene 1 (70 mg,0.1 mmol) and malononitrile (29 mg,0.44 mmol) was added 0.05 mmol of piperidine and the mixture was heated to 50 8C. The formation of the product (3a) was monitored by TLC and the reaction was completed within 8 h. The crude mixture was concentrated under reduced pressure and the product was collected by a vacuum filtration. Alternatively,the product was purified via flash chromatography. Compounds 3b and 3c were synthesized in the similar procedure.
Compound 3a: White solid (50 mg,yield: 55.7%). 1H NMR (500 MHz,CDCl3): δ 7.50 (s,4H),7.29 (s,8H),4.49 (d,4H, J = 13.7 Hz),3.97 (dd,8H,J = 7.3,7.6 Hz),3.34 (d,4H,J = 13.7 Hz), 1.94 (m,8H),1.04 (t,12H,J = 7.5 Hz); 13C NMR (125 MHz,CDCl3): δ 161.6,158.8,135.8,131.7,126.2,113.6,113.0,80.6,77.7,30.7,23.4, 10.2; HR-MS (ESI): m/z calcd. for C56H48N8O4Na+: 919.3691 [M+Na+]; Found: 919.3674. Crystallographic data: [C56H48N8O4]; T = 173(2) K; Mr = 897.02; Monoclinic; space group P2(1)/n; a = 18.0374(6) Å ; b = 14.0132(5) Å ; c = 19.3730(7) Å ; a = γ = 90°; β = 908; V = 4892.5(3) Å 3; Z = 4; ρcalcd = 1.218 g/cm3; crystal size = 0.34 mm × 0.27 mm × 0.20 mm; μ = 0.079 mm -1; reflections collected 56,035; unique reflections 8624; data/restraints/parameters 8624/1/613; GOF on F2 1.018; Rint for independent data 0.0429; final R1 = 0.0584,wR2 = 0.1443; R indices (all data) R1 = 0.0824, wR2 = 0.1636; largest diff. peak and hole: 0.705 and -0.556 e/Å -3.
Compound 3b: White solid (72 mg,yield: 62.0%). 1H NMR (500 MHz,CDCl3): δ 7.42 (s,1H),6.71 (s,2H),4.40 (d,1H, J = 14.0 Hz),3.85 (d,2H,J = 8.0 Hz),3.84 (s,3H),3.79 (s,3H),3.12 (d, 1H,J = 14.0 Hz),1.86 (m,2H),0.97 (t,3H,J = 7.5 Hz); 13C NMR (125 MHz,CDCl3): δ 167.4,164.5,159.1,142.1,135.2,130.2,126.9, 123.1,52.6,52.4,31.0,23.2,10.2; HR-MS (ESI): m/z calcd. for C64H72O20Na+: 1183.4508 [M+Na+]; Found: 1183.4482.
Compound 3c: White solid (70 mg,yield: 68.3%). 1H NMR (500 MHz,CDCl3): δ 7.90 (s,4H),7.36 (s,8H),4.47 (d,4H, J = 13.8 Hz),3.94 (dd,8H,J1 = 7.3 Hz,J2 = 7.4 Hz),3.87 (s,12H),3.32 (d,4H,J = 13.6 Hz),1.91 (m,8H),1.02 (t,J = 7.4 Hz,12H); 13C NMR (CDCl3,125 MHz,ppm): δ 163.2,160.8,154.4,135.6,132.1,126.5, 115.8,100.4,77.3,53.0,30.8,23.4,10.2; HR-MS (ESI): m/z calcd. for C60H61N4O12: 1029.4286 [M+H+]; Found: 1029.4468. 2.2. Synthesis of asymmetric substituted calix[4]arene 4a- c
To a round bottom flask containing a methanol solution (20 mL) of tri-formyl-tetrapropoxycalix[4]arene 2 (810 mg,1.2 mmol) and malononitrile (262 mg,3.96 mmol) was added 0.4 mmol of piperidine and the mixture was heated to 50 ℃. The formation of the product (4a) was monitored by TLC and the reaction was completed within 8 h. The crude mixture was concentrated under reduced pressure and the product was collected by a vacuum filtration. Alternatively,the product was purified via flash chromatography. Compounds 4b and 4c were obtained similarly.
Compound 4a: White solid,yield: 60% (591 mg). 1H NMR (500 MHz,CDCl3): δ 7.60 (s,2H),7.54 (d,2H,J = 1.8 Hz),7.47 (d, 2H,J = 1.7 Hz),7.24 (s,1H),6.90 (s,2H),6.35 (m,3H),4.46 (dd,4H, J = 13.9,13.8 Hz),4.10 (m,2H),3.99 (m,2H),3.87 (t,2H,J = 7.1 Hz), 3.74 (t,2H,J = 7.2 Hz),3.29 (dd,4H,J = 14.7,14.4 Hz),1.89 (m,8H), 1.06 (m,6H),0.96 (t,6H,J = 7.5 Hz); 13C NMR (125 MHz,CDCl3): δ 162.9,161.4,159.1,158.8,155.7,137.7,136.6,135.4,133.0,132.7, 131.4,131.2,128.2,126.0,125.8,122.5,114.0,113.1,112.8,80.1,79.7, 77.6,77.4,77.2,30.9,30.8,23.4,23.3,10.5,10.4,10.0; HR-MS (ESI): m/z calcd. for C52H48N6O4Na+: 843.3630 [M+Na+]; Found: 843.3692.
Compound 4b: White solid,yield: 72.5% (887 mg). 1H NMR (500 MHz,CDCl3): δ 7.52 (s,2H),7.32 (s,1H),6.86 (s,2H),6.82 (s, 2H),6.64 (dd,1H,J = 8.3,11.7 Hz),6.50 (s,2H),6.37 (d,2H, J = 7.0 Hz),4.39 (d,4H,J = 13.7 Hz),3.85 (m,8H),3.79 (m,18H),3.11 (t,4H,J = 14.5 Hz),1.85 (m,8H),1.00 (m,6H),0.93 (t,6H,J = 7.4 Hz); 13C NMR (125 MHz,CDCl3): δ 167.6,167.3,164.9,164.7, 159.8,142.7,142.6,136.5,135.6,134.9,133.8,130.8,130.3,130.1, 128.1,126.7,126.6,122.9,122.5,76.9,76.8,52.7,52.6,52.5,52.4, 31.0,23.3,23.2,10.4,10.3,10.1; HR-MS (ESI): m/z calcd. [M+Na+] for C58H66O16Na+: 1041.4243; Found: 1041.4262.
Compound 4c: White solid,yield: 68.3% (754 mg,). 1H NMR (500 MHz,CDCl3): δ 7.94 (s,1H),7.89 (s,2H),7.37 (s,2H),7.34 (s,2H), 7.28 (s,2H),6.59 (d,2H,J = 7.4 Hz),6.52 (d,1H,J = 7.7 Hz),4.46 (dd, 4H,J = 13.8,13.7 Hz),3.92 (m,14H),3.83 (d,3H,J = 7.6 Hz),3.28 (dd, 4H,J = 13.8,13.7 Hz),1.91 (m,8H),1.01 (m,12H); 13C NMR (125 MHz, CDCl3): δ 163.6,163.4,161.5,161.0,156.1,155.1,154.7,136.7,135.7, 134.0,132.4,132.2,131.7,128.6,126.4,126.0,122.7,115.9,99.8,77.2, 76.9,53.2,53.1,30.9,30.8,23.4,23.4,10.3,10.2; HR-MS (ESI): m/z calcd. for C55H57N3O10Cl -: 954.3727 [M+Cl -]; Found: 954.3582. 3. Results and discussion
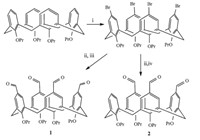
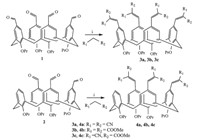
As shown in Scheme 1,tetra-formyltetrapropoxycalix[4]arene (1) and tri-formyltetrapropoxycalix[4]arene (2) were obtained according to literature procedures [8, 9, 10]. The malonate derivatives-calix[4]arene conjugates (3a-3c and 4a-4c) were synthesized through Knoevenagel condensation reaction between formyltetrapropoxycalix[4]arenes (1 and 2) and malonate derivatives in the presence of piperidine in methanol at 50 ℃ in 8 h (Scheme 2).Symmetrically substituted calix[4]arenes 3a-3c were obtained in the yields of 55.7%,62% and 68.3%,respectively. While asymmetrically substituted calix[4]arenes 4a-4c were obtained in the yields of 60%,72.5% and 68.3%,respectively.
|
Download:
|
| Scheme 1. Synthesis of compounds 1 and 2. Conditions: (i) NBS, acetone, r.t.; (ii) n-BuLi, THF, -78 ℃; (iii) excessive dry DMF, HCl; (iv) 3 equiv. dry DMF, HCl. | |
|
Download:
|
| Scheme 2. Synthesis of symmetric and asymmetric substituted calix[4]arene 3a–3c and 4a–4c. Conditions: (i) piperidine, methanol, 50 ℃. | |

|
Download:
|
| Fig. 1.1H NMR of compounds 3a and 4a.. | |
|
Download:
|
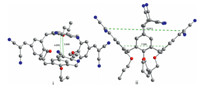
| Fig. 2.X-ray crystal structures of 3a: top view (i), side view (ii). Color code: N (blue),O (red), C (gray). | |
The 1H NMR,13C NMR and mass spectra of 3a-3c and 4a-4c are consistent with the assigned structures. In the 1H NMR spectrum of symmetric 3a,aromatic hydrogens display two singlets at 7.48 and 7.29 ppm,and the bridging methylene protons split into doublets at 4.49 and 3.34 ppm. While the 1H NMR spectrum of asymmetric 4a is more complex than that of the symmetric 3a,the aromatic hydrogens of asymmetric 4a exhibit several singlets,doublets and even multiplets,and the resonance of its bridging methylene protons shows two triplets,as shown in Fig. 1. Similar phenomenon in the 1H NMR spectra of 3b-3c,as well as 4b-4c was observed (see Supporting information).
The structure of 3a was unambiguously established by single crystal X-ray diffraction analysis. Single crystals of 3a have been obtained in mixed solvent of ethyl acetate and petroleum ether. As shown in Fig. 2,calix[4]arene 3a adopts a typical,pinched cone conformation with dihedral angles between the opposing phenoxy rings of 16.4° and 86.6°,respectively,and the centroid-to-centroid distances between the two pairs of opposing phenyl planes are 4.876 Å and 7.555 Å ,respectively. The cyano groups in each malononitrile function are conjugated to the connected benzene ring and only slightly twisted away from the phenyl planes. The distances between the central carbon atoms of the two pairs of face-to-face oriented malononitrile functions are 3.858 Å and 12.911 Å ,respectively. No guest molecule was found being included in the narrow void space. 4. Conclusion
In summary,series of malonate derivatives functionalized calix[4]arenes were synthesized by Knoevenagel condensation of formyl-tetrapropoxycalix[4]arene with corresponding malonate derivatives (malononitrile,methyl 2-cyanoacetate and dimethyl malonate) in good yields. Single crystal X-ray study of malononitrile derived calix[4]arene revealed a pinched cone conformational structure. No guest molecules were found to be included in its narrow cavity. The cyano and ester groups in these calix[4]arene structures might be further transformed to other functional groups,such as amino,amide,carboxyl and hydroxyl groups, which could diversify the structures and functions of the calix[4]arene analogous for potential use in molecular recognition and other related research fields. Research works in these directions are ongoing in our laboratory.
Acknowledgment
Financial support from the National Natural Science Foundation of China (No. 21371177) is acknowledged.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05.017.
| [1] | (a) C.D. Gutsche, Calixarenes: An Introduction, Royal Society of Chemistry, Cambridge, UK, 2008; (b) Z.Q. Shi, Y.Q. Feng, S.X. Meng, Novel synthesis of N-substituted-calix[4]azacrown derivatives, Chin. Chem. Lett. 21 (2010) 807–809; (c) Y. Zhou, H. Li, Y.W. Yang, Controlled drug delivery systems based on calixarenes, Chin. Chem. Lett. 26 (2015) 825–828; (d) H. Li, L.L. Tan, P. Jia, et al., Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods, Chem. Sci. 5 (2014) 2804–2808; (e) Y. Zhou, L.L. Tan, Q.L. Li, et al., Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors, Chem. Eur. J. 20 (2014) 2998–3004. |
| [2] | (a) Y. Sun, F. Zhang, L. Zhang, et al., Synthesis of calix[4]arene derivatives via a Pdcatalyzed Sonogashira reaction and their recognition properties towards phenols, Chin. Chem. Lett. 25 (2014) 226–228; (b) H.H. Zhou, X.M. Shang, Z. Luo, et al., A new member of the calix[4]crown family: facile synthesis and characterization of a calix[4]crown-9 cone conformer, Chin. Chem. Lett. 20 (2009) 143–146; (c) H.F. Wang, Y.B. Zhang, F.J. Huo, C.X. Yin, J.B. Chao, The investigation of inclusion behavior of solvent violet 9 with 4-sulfonatocalix[n]arenes and its recognition to DNA, Chin. J. Chem. 20 (2002) 322–326. |
| [3] | (a) R. Joseph, C.P. Rao, Ion and molecular recognition by lower rim 1,3-di-conjugates of calix[4]arene as receptors, Chem. Rev. 111 (2011) 4658–4702; (b) E.V. Ukhatskaya, S.V. Kurkov, S.E. Matthews, Encapsulation of drug molecules into calix[n]arene nanobaskets, role of aminocalix[n]arenes in biopharmaceutical field, J. Pharm. Sci. 102 (2013) 3485–3512; (c) F. Corbellini, R. Fiammengo, P. Timmerman, et al., Guest encapsulation and self-assembly of molecular capsules in polar solvents via multiple ionic interactions, J. Am. Chem. Soc. 124 (2002) 6569–6575. |
| [4] | (a) H.Y. Guo, F.F. Yang, Z.Y. Jiao, J.R. Lin, Click synthesis and dye extraction properties of novel thiacalix[4]arene derivatives with triazolyl and hydrogen bonding groups, Chin. Chem. Lett. 24 (2013) 450–452; (b) L. Baldini, A. Casnati, F. Sansonea, R. Ungaro, Calixarene-based multivalent ligands, Chem. Soc. Rev. 36 (2007) 254–266; (c) M.G.J. ten Cate, D.N. Reinhoudt, M. Crego-Calama, Binding of small guest molecules to multivalent receptors, J. Org. Chem. 70 (2005) 8443–8453. |
| [5] | (a) D.V. Dervartanian, C. Veeger, Studies on succinate dehydrogenase. I. Spectral properties of the purified enzyme and formation of enzyme-competitive inhibitor complexes, Biochim. Biophys. Acta 92 (1964) 233–247; (b) G.D. Zeevalk, E. DerrYellin, W.J. Nicklas, Relative vulnerability of dopamine and GABA neurons in mesencephalic culture to inhibition of succinate dehydrogenase by malonate and 3-nitropropionic acid and protection by NMDA receptor blockade, J. Pharmacol. Exp. Ther. 275 (1995) 1124–1130; (c) J.G. Greene, J.T. Greenamyre, Characterization of the excitotoxic potential of the reversible succinate dehydrogenase inhibitor malonate, J. Neurochem. 64 (1995) 430–436. |
| [6] | S.Y. Li, Y.W. Xu, S.Q. Zeng, et al., Highly selective fluorescent calix[4]arene chemosensor for acidic amino acids in pure aqueous media, Tetrahedron Lett. 53 (2012) 2918–2921. |
| [7] | (a) S.T. Zhang, K. Cheng, X.H. Wang, H. Yin, Selection, synthesis and anti-inflammatory evaluation of the arylidene malonate derivatives as TLR4 signaling inhibitors, Bioorg. Med. Chem. 20 (2012) 6073–6079; (b) A. Kaya, H.K. Alpoguz, A. Yilmaz, Application of Cr(VI) transport through the polymer inclusion membrane with a new synthesized calix[4]arene derivative, Ind. Eng. Chem. Res. 52 (2013) 5428–5436; (c) A. Dondoni, A. Marra, Calixarene and calixresorcarene glycosides: their synthesis and biological applications, Chem. Rev. 110 (2010) 4949–4977. |
| [8] | C.D. Gutsche, M. Iqbal, p-tert-Butylcalix[4]arene, Org. Synth. 68 (1990) 234. |
| [9] | C.D.Gutsche, J.A. Levine, Calixarenes. 6. Synthesis of a functionalizable calix[4]arene in a conformationally rigid cone conformation, J. Am. Chem. Soc. 104 (1982) 2652–2653. |
| [10] | M.S. Wong, Z.H. Li, C.C. Kwok, Highly extended styrylstyrylcalix[4]arene assemblies: synthesis and optical properties, Tetrahedron Lett. 41 (2000) 5719–5723. |




