Cyclodextrins (CDs) have a unique structural characteristic,the outer surface is hydrophilic and the inner cavity is hydrophobic. CDs are able to form host-guest complexes with many surfactants [1, 2, 3],which has become a focus of attention in self-assembly research. Jang [4, 5, 6, 7, 8] designed and performed a series of experiments with CD-based host-guest systems,and found that CDs served as destructive modulators,constructive modulators,and unamphiphilic building units. At the same time,the microstructures of surfactant molecule self-organized assemblies under different conditions are academically researched by means of NMR spectroscopy,small- and wide-angle X-ray scattering (SAXS, WAXS),neutron scattering,infrared spectroscopy,confocal laser scanning microscopy (CLSM),atomic force microscopy (AFM), freeze-fracture transmission electron microscopy (FF-TEM),and so on [9, 10, 11, 12, 13, 14, 15]. For instance,the structure transition of SDS/β-CD complexes,from vesicles to microtubules to lamellae with increasing concentration,was characterized by AFM,CLSM,SAXS, WAXS and TEM [14, 15].
Besides those methods listed above,dielectric relaxation spectroscopy (DRS) is also considered an effective and noninvasive way to investigative the dynamic properties and microstructures of surfactant systems [16, 17, 18, 19, 20, 21]. For example,Redwood found that phosphatidylcholine vesicle exhibited relaxation at 40 MHz [22], and Barchini studiedthe electro-dynamic properties of the liposome suspension theoretically and experimentally [23]. However,there are seldom reports about vesicle and liquid crystal systems characterized by DRS,and most research focuses on phosphatidylcholine vesicles and liposomes. This paper studied the dielectric relaxation behavior of SDS/β-CD organized assembly aqueous solutions in the 1%-7% concentration range,obtained the concentration dependence of the system’s different internal microstructures, and characterized SDS/β-CD organized assemblymorphology and interface migration by means of relaxation behavior. 2. Experimental
Sodium dodecyl sulfate (SDS,99%) and β-cyclodetrin (β-CD, containing 14% water) were purchased from Sinopharm Chemical Reagent Co. Ltd. Deionized water was prepared by an Aquapro P Series water purification system (Taiwan). The mass concentration range of SDS/β-CD aqueous solutions was from 1.0% to 7.0% (a constant SDS and β-CD molar ratio of 1:2). Dielectric measurements were carried out on a WK6500B Precision Impedance Analyzer (Wayne Kerr) over a continuous frequency range of 100- 80 MHz. The amplitude of the applied alternating field was 500 mV. A dielectric measurement cell with concentrically cylindrical platinum electrodes was employed,and the volume of the solution used in the experiment was about 20 mL in order to ensure the electrodes were submerged. The temperature of the cell was 25±0.5℃,which was controlled by circulating thermo-stated water.
Dielectric relaxation spectroscopy measured the polarization of the sample by an applied electric field of frequency f. The complex permittivity spectrum can indicate the sample’s polarization. In order to obtain the parameters of dielectric relaxation,the Cole- Cole empirical equation is used to fit the experimental data. Because the effect of electrode polarization on the electrode surface often obscures the dielectric relaxation when it is measured in the low-frequency range,the electrode polarization term is added to the Cole-Cole equation [24]. In this way,the effect of electrode polarization can be subtracted from the experimental data,and the real dielectric response of the investigated samples is achieved. The Cole-Cole equation with electrode polarization term is the following
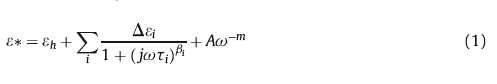
Fig. 1 shows the three-dimensional representations of the concentration-dependent relative permittivity and conductivity spectra at 25℃ as the mass concentration of SDS/β-CD solution transfers from 1% to 7%. For concentrations up to about 4%,a dielectric relaxation can be found in Fig. 1. In order to investigate the phase micro-structure and transition of SDS/β-CD solutions with concentration increasing from 1% to 7%,Eq.1 was used to fit the dielectric parameters of SDS/β-CD solution with different mass concentrations. The fitting result was listed in Table 1 and Fig. 2. At lower concentrations,both ε and κ continuously increased with concentration. With the concentration increasing above 4%,ε started to increase quickly as shown by the arrow in Fig. 1a. Different from ε,meanwhile,κ increased with concentration from 1% to 3.5%,and then began to decrease as shown by the in Fig. 1b.
| Table 1 Dielectric parameters of SDS/β-CD solution with different concentrations. |
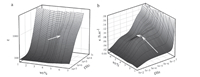
|
Download:
|
| Fig. 1.3D representations of the mass concentration dependence of the relative permittivity spectrum (a) and the conductivity spectrum (b) of the SDS/β-CD solution at 25℃. | |
|
Download:
|
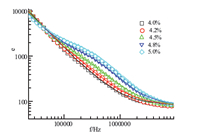
| Fig. 2.Dielectric constant spectra of different SDS/β-CD mass concentrations fitted with Cole-Cole equation. Symbols represent the experimental data and solid lines represent the best fitting curves evaluated from Eq. (1). | |
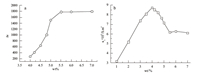
Fig. 3 shows the SDS/β-CD mass concentrations dependency of dielectric increment Δε(a) and low frequency conductivity κl (b). Δε increases with concentration from 4% to 5.5% and stays constant at concentrations above 5.5%. Low frequency conductivity increased with concentration up to a concentration value of 4%,while the low frequency conductivity decreased as the concentration increased in the range of 4%-5.5%. When the mass concentration was greater than 5.5%,the low frequency conductance changed a little. According to the fitted dielectric parameters, the dielectric increment Δε (a),and the low frequency conductivity κl,phase behavior transition can be roughly separated into three stages,below 4%,from 4% to 5.5%,and above 5.5%. The result is similar with Jiang’s work [14].
Under the usual frequency range (from 100 Hz to 1×108Hz), the characteristic dielectric relaxations in a heterogeneous structure of dispersing systems appeared at several megahertz, which originates from the Maxwell-Wagner (M-W) interfacial polarization effect [25]. According to the fitted data in Table 1,the characteristic relaxation frequencies (f0) are near megahertz,the dielectric relaxation of SDS/β-CD solution should be ascribed to the M-W interfacial polarization,and the presence of vesicle and water phase interfaces was indicated. According to the data in Table 1,the mean β of SDS/β-CD solution is 0.9. According to the Cole-Cole equation,if the parameter β is close to 1,the dielectric relaxation is considered as an ideal Debye relaxation and the dielectric relaxation is dominated by a single relaxation mechanism. If β is far from 1,it means that the relaxation is associated with multiple relaxation mechanisms. So,for concentrations from 4% to 7%,the dielectric relaxation is considered as an ideal Debye relaxation. The dielectric increment can reflect the degree of system interface polarization,so it is closely related to the assembly number and the interface electrical properties.
Dielectric increment increased gradually to a constant value as the solution mass concentration increased to 5.5%. This is thought to be caused by the increasing dissociation number of Na+ ions with increasing SDS/β-CD mass concentration,which also increases the concentration of counter-ions which formed tangential migration of the surface bound layer. We knew that the value of β had been used to forecast the shape or morphology of the aggregates [16],and we see β began to decrease in Table 1, indicating that the aggregates start to transition from a vesicular to a microtubule arrangement as the concentration exceeded 5.5%. Because the SDS/β-CD system began to form vesicles in 4%,and counter-ions bonded to the vesicle interior,κl began to decrease. While the SDS/β-CD system formed microtubules,κl no longer decreased,because the counter-ions unbound from the interface.
This experiment covers three distinct phases as the mass concentration increased from 1% to 7%: (1) SDS and β-CD monomers and SDS/β-CD complexes including (2) vesicles,and (3) microtubules. In order to better understand the transformation process,Fig. 4 displays the transition process of SDS/β-CD shape with concentration from 1% to 7%. 4. Conclusion
SDS/β-CD aqueous solutions exhibited dielectric relaxation as the mass concentration increased from 4% to 7%. The dielectric relaxation of SDS/β-CD solution was assigned to the M-W interfacial polarization. The relaxation parameter reflected the shape transition from vesicles to microtubules with increasing concentration. Our work provided a new method to study surfactant organized assembly.
|
Download:
|
| Fig. 3.SDS/β-CD mass concentrations dependency of dielectric increment Δε(a) and low frequency conductivity κl (b). | |

|
Download:
|
| Fig. 4.The transition process of the SDS/β-CD shape with the concentration increasing [14]. | |
Dielectric relaxation method study on the present SDS/β-CD aqueous solution is still in its infancy,and there are some issues that have not been fully addressed. For example,although we know that SDS/β-CD vesicle belongs to M-W interfacial polarization, we have not obtained other parameters like radius and surface charge density. Further,whether or not the dielectric method can be applied to other vesicle systems has not been determined.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21203005,21376009,21176004),the National Key Technologies R&D Program of China for the 12th Five- Year Plan (No. 2013BAC01B04).
| [1] | A.B. Dorrego, L. García-Río, P. Hervé s, Micellization versus cyclodextrin–surfactant complexation, Angew. Chem. Int. Ed. 39 (2000) 2945–2948. |
| [2] | A.J. Valente, O. Söderman, The formation of host–guest complexes between surfactants and cyclodextrins, Adv. Colloid Interface Sci. 205 (2014) 156–176. |
| [3] | L. Jiang, Y. Yan, J. Huang, Selectivity and stoichiometry boosting of β-cyclodextrin in cationic/anionic surfactant systems: when host–guest equilibrium meets biased aggregation equilibrium, J. Phys. Chem. B 114 (2010) 2165–2174. |
| [4] | Y. Yan, L. Jiang, J. Huang, Unveil the potential function of CD in surfactant systems, Phys. Chem. Chem. Phys. 13 (2011) 9074–9082. |
| [5] | L.X. Jiang, M. Deng, Y. Wang, Special effect of β-cyclodextrin on the aggregation behavior of mixed cationic/anionic surfactant systems, J. Phys. Chem. B 113 (2009) 7498–7504. |
| [6] | L.X. Jiang, Y. Yan, J. Huang, Selectivity and stoichiometry boosting of β-cyclodextrin in cationic/anionic surfactant systems: when host–guest equilibrium meets biased aggregation equilibrium, J. Phys. Chem. B 114 (2010) 2165–2174. |
| [7] | L.X. Jiang, Y. Yan, J. Huang, Zwitterionic surfactant/cyclodextrin hydrogel: microtubes and multiple responses, Soft Matter 7 (2011) 10417–10423. |
| [8] | L.X. Jiang, Y. Yan, J. Huang, Versatility of cyclodextrins in self-assembly systems of amphiphiles, Adv. Colloid Interface Sci. 169 (2011) 13–25. |
| [9] | L. Zheng, M. Suzuki, T. Inoue, Aqueous phase behavior of hexaethylene glycol dodecyl ether studied by differential scanning calorimetry, Fourier transform infrared spectroscopy, and 13C NMR spectroscopy, Langmuir 18 (2002) 9204–9210. |
| [10] | A. Tonegawa, K. Ohno, H. Matsuura, A combined Raman and deuterium NMR spectroscopic study on the molecular and phase structure of a nonionic surfactant c12e5-water system, J. Phys. Chem. B 106 (2002) 13211–13223. |
| [11] | H. Walderhaug, K.D. Knudsen, Microstructures in aqueous solutions of a polyoxyethylene trisiloxane surfactant and a co-surfactant studied by SANS and NMR self-diffusion, Langmuir 24 (2008) 10637–10645. |
| [12] | D.J.F. Taylor, R.K. Thomas, J. Penfold, Polymer/surfactant interactions at the air/water interface, Adv. Colloid Interface Sci. 132 (2007) 69–110. |
| [13] | P. Gianni, L. Bernazzani, R. Carosi, Micellization of lithium perfluoroheptanoate and its aggregation on poly (ethylene glycol) oligomers in water, Langmuir 23 (2007) 8752–8759. |
| [14] | L.X. Jiang, Y. Peng, Y. Yan, Aqueous self-assembly of SDS@2β-CD complexes: lamellae and vesicles, Soft Matter 7 (2011) 1726–1731. |
| [15] | L.X. Jiang, Y. Peng, Y. Yan, Annular Ring microtubes formed by SDS@2β-CD complexes in aqueous solution, Soft Matter 6 (2010) 1731–1736. |
| [16] | L.K. Yang, K.S. Zhao, J.X. Xiao, Study of tetrabutylammonium perfluorooctanoate aqueous solutions with two cloud points by dielectric relaxation spectroscopy, Langmuir 22 (2006) 8655–8662. |
| [17] | L. Yang, K. Zhao, Dielectric model and theoretical analysis of cationic reverse micellar solutions in CTAB/isooctane/n-hexanol/water systems, Langmuir 23 (2007) 8732–8739. |
| [18] | W. Schrader, S. Halstenberg, R. Behrends, U. Kaatze, Critical slowing in lipid bilayers, J. Phys. Chem. B 107 (2003) 14457–14463. |
| [19] | Y.W. Zhou, W. Zhou, F. Han, Dielectric analysis on phase transition and micelle shape of polyoxyethylene trisiloxane surfactant in dilute aqueous solution, Chin. Chem. Lett. 22 (2011) 745–748. |
| [20] | Y. Lian, K. Zhao, Study of micelles and microemulsions formed in a hydrophobic ionic liquid by a dielectric spectroscopy method. I. Interaction and percolation, Soft Matter 7 (2011) 8828–8837. |
| [21] | L. Ma, K. Zhao, Dielectric relaxation spectroscopy for the binary system of 1-butyl- 3-methylimidazolium hexafluorophosphate and ethanol: interactions and micro phase behavior, RSC Adv. 2 (2012) 10007–10014. |
| [22] | W.R. Redwood, S. Takashima, H.P. Schwan, T.E. Thompson, Dielectric studies on homogeneous phosphatidylcholine vesicles, Biochim. Biophys. Acta 255 (1972) 557–566. |
| [23] | R. Barchini, H.P. Van Leeuwen, J. Lyklema, Electrodynamics of liposome dispersions, Langmuir 16 (2000) 8238–8247. |
| [24] | K. Asami, Dielectric relaxation in a water-oil-triton X-100 microemulsion near phase inversion, Langmuir 21 (2005) 9032–9037. |
| [25] | K. Asami, Characterization of heterogeneous systems, Prog. Polym. Sci. 27 (2002) 1617–1659. |