b Department of Theoretical Chemistry and Biology, School of Biotechnology, KTH Royal Institute of Technology, Stockholm SE-10691, Sweden
Since the first report by Grätzel [1],dye-sensitized solar cells (DSSCs) have attracted increasing attention in utilizing solar energy because of their relatively low cost,easy fabrication,and environmentally friendly character [2, 3, 4, 5, 6]. In recent years,many groups are devoted to the development of novel dyes to improve cell efficiencies. In this respect,porphyrins have been demonstrated to be promising,because of their high molar absorption coefficients, good photostability,beautiful color,as well as high cell efficiencies. In addition,the properties can be easily modulated by the peripheral substituents and inner coordinated metal ions [7]. Thus,D-π-A type porphyrins have been intensively investigated for developing efficient DSSC dyes. Especially,porphyrins containing meso-diphenylamine donor groups exhibited relatively high cell efficiencies [8, 9]. For example,Grätzel and co-workers have reported the highest DSSC efficiency of 13.0% for porphyrin dyes containing diphenylamine donors and extended benzoic acid acceptors [10].
In addition to designing efficient porphyrin dyes,cosensitization with another organic dye is also effective in further improving the efficiencies of porphyrin-based DSSCs [11, 12, 13]. In this respect, our group recently reported porphyrin dyes with carbazole-based electron donors,in combination with the cosensitization approach, achieving the high cell efficiency of 10.75% [11, 12].
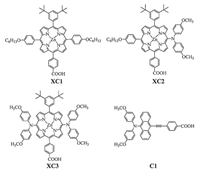
Herein,we report the investigations on the effect of numbers and positions of the diphenylamine units on the DSSC efficiencies, with the purpose to further understand the efficiency-structure correlations. Thus,porphyrin dyes XC1-XC3 were synthesized by introducing various numbers of bis(4-methoxyphenyl)amino and p-hexyloxyphenyl groups as the donors (Scheme 1),and the effect of dye structures on the efficiencies was investigated. To our delight,introduction of the bis(4-methoxyphenyl)amino unit can effectively enhance the light harvesting around 500 nm,and the cell efficiencies are highly dependent on the donors. By further application of the cosensitizer C1 (Scheme 1),a cell efficiency of 5.75% was achieved.
|
Download:
|
| Scheme 1.Molecular structures of porphyrin dyes XC1–XC3 and cosensitizer C1. | |
All reagents and solvents were obtained from commercial sources and used without further purification unless otherwise noted. The transparent FTO conducting glass (fluorine-doped SnO2, transmission > 90% in the visible range,sheet resistance 15 Ω/ square) and the TiO2 paste was purchased from Geao Science and Educational Co.,Ltd. The FTO conducting glass was washed with a detergent solution,deionized water,acetone,and ethanol successively under ultrasonication for 20 min before use,respectively. The synthesis of C1 has been communicated [12]. The synthetic details,computational methods and photovoltaic measurements are described in the Supporting Information. 3. Results and discussion
As mentioned above,diphenylamino units may be introduced into the meso-positions of porphyrins as strong electron donors to synthesize efficient DSSC dyes. Meanwhile,p-hexyloxyphenyl groups not only can be used as weak electron donors,but also can be employed for suppressing the dye aggregation effect due to the presence of long alkyl chains [14]. Therefore,for the purpose of modulating molecular energy levels and suppressing dye aggregation,various numbers of bis(4-methoxyphenyl)amino and p-hexyloxyphenyl groups were introduced to afford the target porphyrin dyes XC1-XC3 (Scheme 1,S1 and Figs. S1-S18 in Supporting information). 3.1. Spectral properties
Wide and strong absorption characteristics are favored for capturing the solar light. Dyes XC1-XC3 exhibit the typical features of porphyrins,with an intense Soret band in the range of 400-450 nm,and less intense Q bands in a range of 500-750 nm (Fig. 1 and Table S1 in Supporting information). Compared with XC1,dyes XC2 and XC3 exhibit new absorption bands centered at ca. 485 nm with increasing intensities,which may be ascribed to the charge transfer associated with the strongly electron donating bis(4-methoxyphenyl)amino groups. Furthermore,the Q bands and emission wavelengths of XC2 and XC3 are successively redshifted (Fig. 1),indicating that they may have stronger light harvesting ability in the long wavelength range,which is favorable for improving the cell efficiencies.

|
Download:
|
| Fig. 1.(a) Absorption spectra and (b) emission spectra of porphyrin dyes XC1–XC3 in CHCl3–CH3OH (v/v, 2/1). | |
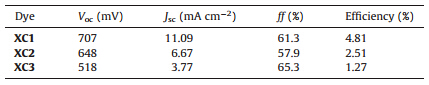
| Table 1 Photovoltaic parameters of DSSCs based on XC1–XC3. |
Compared with the corresponding solution spectra,absorption bands of the dyes loaded TiO2 films are broadened (Fig. 2),which will result in increased light-harvesting ability. Dye XC1 shows split Soret bands. The red-shifted one results from the J-aggregation of the dye molecules on the TiO2 films [15],and the blueshifted one can be ascribed to the deprotonation of the carboxylic acid group upon dye attachment to the TiO2 surface [16, 17],and/or the H-aggregation of the dyes [18, 19].
In contrast to XC1,dye XC2 shows a slightly blue-shifted Soret band when it is loaded onto the TiO2 film,and no obvious change was observed for the Soret band of XC3 upon loading onto TiO2.

|
Download:
|
| Fig. 2.Normalized UV–vis spectra of dyes XC1–XC3 in CHCl3–CH3OH (v/v, 2/1) and on TiO2 films. | |
To effectively realize the electron injection and dye regeneration processes in DSSCs,HOMO and LUMO orbitals of the dyes should be aligned at suitable energy levels. Thus,the cyclic voltammograms for XC1-XC3 were recorded in CH2Cl2 (Fig. 3),and the corresponding data are summarized in Table S2 in Supporting information. It can be obtained from Fig. 3 that the first oxidation potentials for XC1-XC3 are 0.70,0.55,0.49 V,respectively. The E0-0 energy gap values estimated from the absorption threshold of the dyes adsorbed on the TiO2 films turn out to be 1.98,1.76,1.69 V for XC1-XC3,respectively. Thus,the LUMO levels of XC1-XC3 can be calculated to be -1.28,-1.21,-1.18 V,respectively. These values are all significantly higher than the conduction band (CB) of the TiO2 electrode,and thus enabling the electron injection processes. On the other hand,the HOMO level for XC1 is obviously lower than the I-/I3- energy level,which makes the dye regeneration process feasible. Hence,XC1 is suitable for fabrication of efficient DSSCs in terms of the energy levels. In contrast,the HOMO levels for XC2 and XC3 are elevated too much by the strongly electron donating bis(4-methoxyphenyl) amino groups [5],and thus the dye regeneration process may be suppressed,which is unfavorable for the DSSC efficiencies.

|
Download:
|
| Fig. 3.(a) Cyclic voltammetry plots and (b) energy-level diagram of the dyes adsorbed to TiO2 films. | |
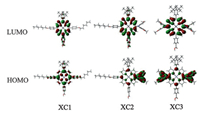
To gain further insight into the electron distribution of the frontier molecular orbitals,density functional theory (DFT) calculations and time dependent DFT (TD-DFT) calculations were performed. Fig. 4 shows the corresponding molecular orbital profiles. For XC1,the HOMO is mainly delocalized over the porphyrin framework,and the LUMO is distributed over the porphyrin framework and the carboxyl group. Dye XC2 has a stronger electron donor attached to the porphyrin framework, with the HOMO mainly localized over the donor,and the LUMO is delocalized over the porphyrin macrocycle and the carboxyl anchoring group. The electron distribution profiles imply that intramolecular charge separation states may be possible for both XC1 and XC2,accompanied with electron transfer from the donors or the porphyrin macrocycles to the anchoring moieties,thus enabling the electron injection processes.
|
Download:
|
| Fig. 4.Frontier molecular orbital profiles of dyes XC1–XC3. | |
However,the LUMO of dye XC3 is localized within the porphyrin macrocycle,and isolated from the carboxyl anchoring moiety (Fig. 4),indicating that the excited state electron of XC3 cannot be efficiently transferred to the conduction band of TiO2, and thus a low Jsc value and a poor power conversion efficiency are expected for XC3 [14, 20],which are consistent with the photovoltaic results (vide infra). 3.4. Photovoltaic performance
From above mentioned results,it can be anticipated that all the three porphyrin dyes may be employed as DSSC sensitizers. Thus, the DSSC devices were fabricated using XC1-XC3 respectively as the sensitizers,and the photovoltaic behavior was investigated (Table 1 and Fig. 5b). The overall power conversion efficiencies lie in the order of XC1 > XC2 > XC3. The XC1-based DSSCs exhibit the highest power conversion efficiency of 4.81%,while the efficiency of XC3-based DSSC is only 1.27%.

|
Download:
|
| Fig. 5.(a) IPCE spectra and (b) current–voltage characteristics of DSSCs based on dyes XC1–XC3. | |
The differences of the DSSC device performance can be rationalized in terms of the short-circuit photocurrent density (Jsc) and open-circuit photovoltage (Voc) values. As we know,Jsc is dependent on the IPCE action spectra. Fig. 5a shows that dye XC1 has relatively high IPCE values in the wavelength range of 350- 630 nm,and the values exceed 60% in the spectral ranges of 392-459 nm and 553-570 nm. Whereas,the IPCE values of XC2 and XC3 barely exceed 30%. The lower IPCE and Jsc values for XC2 may be ascribed to insufficient dye regeneration driving force caused by the elevated HOMO orbital. For XC3,the evaluated HOMO and isolation of the LUMO from the carboxyl moiety cooperatively lead to the lowest IPCE values. Consistent with the IPCE spectra,the Jsc values are drastically decreased from 11.09 to 6.67 and 3.77 mA cm-2 from XC1 to XC2 and XC3.
The Voc values for DSSCs are related to the electron transport and recombination processes. Thus,electrochemical impedance spectral (EIS) analyses were performed for the DSSCs based on dyes XC1-XC3 (Fig. 6). In the Nyquist plots,usually two semicircles can be observed,with the second one corresponding to the electron transport process at the TiO2/dye/electrolyte interface [21, 22]. In Fig. 6a,the 1st semicircles for all the dyes are very small,and the radii of the 2nd ones lie in the order of XC3 < XC2 < XC1, indicating the sequence of charge transfer resistance at the interface,which are consistent to the order of Voc for XC1-XC3. The DSSC device based on XC1 demonstrates the most effectively suppressed charge recombination process,which may be ascribed to the retardation of the diffusion of I3- ions into the nanoporous TiO2 electrode by the long hexyloxyl chains. On the other hand, the electron lifetimes [23, 24] of the devices based on XC1-XC3 are calculated from the Bode plots (Fig. 6b) to be 37.9,19.6,and 14.5 ms,respectively. The longest electron lifetime observed for XC1 is indicative of effective suppression of the back reaction of the injected electron with the I3- in the electrolyte,resulting in the highest Voc. In summary,the EIS results are in good agreement with the photovoltaic performance.

|
Download:
|
| Fig. 6. Electrochemical impedance spectra. (a) Nyquist plots; (b) Bode phase plots of DSSCs based on dyes XC1–XC3 (under -0.65 V applied bias voltage in dark). | |
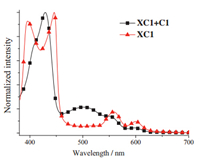
According to the above results,the XC1-based device exhibits a relatively high power conversion efficiency of 4.81%. However,dye XC1 exhibits very poor absorption around 500 nm. To overcome this absorption deficiency,dye C1 [12] was employed for further improving the device performance using the cosensitization approach [12, 25]. As we previously reported [12],C1 exhibits high molar absorption coefficients around 500 nm,and thus it may be a suitable cosensitizer for XC1. Compared with the absorption spectrum of XC1 on the TiO2 film,the absorption spectrum of XC1 and C1 coadsorbed TiO2 film indeed demonstrates a new band centered at 500 nm (Fig. 7).
|
Download:
|
| Fig. 7. Normalized UV–vis spectra of dyes XC1 and XC1 + C1 on TiO2 films. | |
Based on above mentioned results of photophysical properties, DSSC devices were fabricated using C1 as the cosensitizer for XC1, and the efficiency was found to be improved from 4.81% to 5.75% (Fig. 8 and Table S3 in Supporting information). Upon cosensitization,the Jsc value is increased from 11.09 mA cm-2 to 13.44 mA cm-2,because the IPCE valley around 500 nm for XC1 is well filled up (Fig. 8a). Meanwhile,the Voc value is slightly improved,which is consistent with the observation that the cosensitized device has larger charge resistance than that of XC1 (Fig. S19 in Supporting information). Based on these results,it can be concluded that cosensitization of XC1 with C1 is effective for elevating the DSSC efficiency by improving both of Voc and Jsc.

|
Download:
|
| Fig. 8. (a) IPCE spectra and (b) current–voltage characteristics of DSSCs based on XC1 and XC1 + C1. | |
In this work,we have designed and synthesized porphyrin dyes XC1-XC3 with various numbers of p-hexyloxyphenyl and bis(4- methoxyphenyl)amino groups as the electron donors. Among them,XC1 contains two weakly electron donating p-hexyloxyphenyl groups. In contrast,XC2 and XC3 contain strongly electron donating bis(4-methoxyphenyl)amino groups. As a result,the light harvesting around 500 nm was effectively enhanced. However,the highest occupied molecular orbitals (HOMO) for both XC2 and XC3 are elevated too much and thus the dye regeneration processes are suppressed,and isolation of the LUMO from the carboxyl anchoring moiety was observed for XC3, thus leading to poor power conversion efficiencies of 2.51% and 1.27% for XC2 and XC3,respectively. These values are much lower than that of 4.81% for XC1. In addition,cosensitization of XC1 with a non-porphyrin dye C1 elevates the efficiency to 5.75% with improved Jsc and Voc values.
These results provide further insights into the approaches of improving DSSC efficiencies through design of efficient porphyrin dyes as well as cosensitization.
Acknowledgments
This work was financially supported by NSFC/China (Nos. 21472047 and 91227201),the Oriental Scholarship,and NCET-11- 0638.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04.021.
| [1] | B. O'Regan, M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature 353 (1991) 737–740. |
| [2] | Z.L. Ku, X. Li, G.H. Liu, et al., Transparent NiS counter electrodes for thiolate/ disulfide mediated dye-sensitized solar cells, J. Mater. Chem. A 1 (2013) 237–240. |
| [3] | Z. Li, Q.Q. Li, Molecular engineering and cosensitization for developing efficient solar cells based on porphyrin dyes with an extended π-framework, Sci. China Chem. 57 (2014) 1491. |
| [4] | J. Zeng, T.L. Zhang, X.F. Zang, et al., D-A-π-A organic sensitizers containing a benzothiazole moiety as an additional acceptor for use in solar cells, Sci. China Chem. 56 (2013) 505–513. |
| [5] | Y.R. Gao, L.L. Chu, W. Guo, T.L. Ma, Synthesis and photoelectric properties of an organic dye containing benzo[1,2-b:4,5-b']dithiophene for dye-sensitized solar cells, Chin. Chem. Lett. 24 (2013) 149–152. |
| [6] | S.Y. Qu, J.L. Hua, H. Tian, New D-π-A dyes for efficient dye-sensitized solar cells, Sci. China Chem. 55 (2012) 677–697. |
| [7] | L.L. Li, E.W.G. Diau, Porphyrin-sensitized solar cells, Chem. Soc. Rev. 42 (2013) 291–304. |
| [8] | C.L. Wang, Y.C. Chang, C.M. Lan, et al., Enhanced light harvesting with π-conjugated cyclic aromatic hydrocarbons for porphyrin-sensitized solar cells, Energy Environ. Sci. 4 (2011) 1788–1795. |
| [9] | S.L. Wu, H.P. Lu, H.T. Yu, et al., Design and characterization of porphyrin sensitizers with a push-pull framework for highly efficient dye-sensitized solar cells, Energy Environ. Sci. 3 (2010) 949–955. |
| [10] | S. Mathew, A. Yella, P. Gao, et al., Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers, Nat. Chem. 6 (2014) 242–247. |
| [11] | X. Sun, Y.Q. Wang, X. Li, et al., Cosensitizers for simultaneous filling up of both absorption valleys of porphyrins: a novel approach for developing efficient panchromatic dye-sensitized solar cells, Chem. Commun. 50 (2014) 15609–15612. |
| [12] | Y.Q. Wang, B. Chen, W.J. Wu, et al., Efficient solar cells sensitized by porphyrins with an extended conjugation framework and a carbazole donor: from molecular design to cosensitization, Angew. Chem. Int. Ed. 53 (2014) 10779–10783. |
| [13] | A. Yella, H.W. Lee, H.N. Tsao, et al., Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency, Science 334 (2011) 629–634. |
| [14] | B. Chen, X. Li, W.J. Wu, Q.Z. Zha, Y.S. Xie, A novel trigeminal zinc porphyrin and corresponding porphyrin monomers for dye-sensitized solar cells, RSC Adv. 4 (2014) 10439–10449. |
| [15] | J.X. He, W.J. Wu, J.L. Hua, et al., Bithiazole-bridged dyes for dye-sensitized solar cells with high open circuit voltage performance, J. Mater. Chem. 21 (2011) 6054–6062. |
| [16] | Y.S. Yen, C.T. Lee, C.Y. Hsu, et al., Benzotriazole-containing D-π-A conjugated organic dyes for dye-sensitized solar cells, Chem. Asian J. 8 (2013) 809–816. |
| [17] | K.R.J. Thomas, Y.C. Hsu, J.T. Lin, et al., 2,3-Disubstituted thiophene-based organic dyes for solar cells, Chem. Mater. 20 (2008) 1830–1840. |
| [18] | C.L. Wang, C.M. Lan, S.H. Hong, et al., Enveloping porphyrins for efficient dyesensitized solar cells, Energy Environ. Sci. 5 (2012) 6933–6940. |
| [19] | C.F. Lo, S.J. Hsu, C.L. Wang, et al., Tuning spectral and electrochemical properties of porphyrin-sensitized solar cells, J. Phys. Chem. C 114 (2010) 12018–12023. |
| [20] | B. Liu, W.Q. Li, B. Wang, et al., Influence of different anchoring groups in indoline dyes for dye-sensitized solar cells: electron injection, impedance and charge recombination, J. Power Sources 234 (2013) 139–146. |
| [21] | B. Liu, W.H. Zhu, Y.Q. Wang, et al., Modulation of energy levels by donor groups: an effective approach for optimizing the efficiency of zinc-porphyrin based solar cells, J. Mater. Chem. 22 (2012) 7434–7444. |
| [22] | Y.Q. Wang, X. Li, B. Liu, et al., Porphyrins bearing long alkoxyl chains and carbazole for dye-sensitized solar cells: tuning cell performance through an ethynylene bridge, RSC Adv. 3 (2013) 14780–14790. |
| [23] | Z.H. Wang, M. Liang, L.N. Wang, et al., New triphenylamine organic dyes containing dithieno[3,2-b:2',3'-d]pyrrole (DTP) units for iodine-free dye-sensitized solar cells, Chem. Commun. 49 (2013) 5748–5750. |
| [24] | Y.Q. Wang, L. Xu, X.D. Wei, et al., 2-Diphenylaminothiophene as the donor of porphyrin sensitizers for dye-sensitized solar cells, New J. Chem. 38 (2014) 3227–3235. |
| [25] | S. Chang, H.D. Wang, Y. Hua, et al., Conformational engineering of co-sensitizers to retard back charge transfer for high-efficiency dye-sensitized solar cells, J. Mater. Chem. A 1 (2013) 11553–11558. |