The chemistry of macrocyclic polyamines has been received much attention due to their widespread applications in coordination chemistry,medicinal chemistry,biochemistry,and material chemistry [1, 2, 3, 4]. Because of their protonation property at physiological conditions,macrocyclic polyamines often act as positive-charged units in constructing DNA condensation agents and non-viral gene vectors [5, 6, 7, 8]. As a binding unit,macrocyclic polyamines have been frequently applied in the development of artificial nucleases [9, 10, 11, 12] and a number of chemical sensors to monitor biologically- and environmentally-relevant metal and anion ions [13, 14, 15, 16]. Our group has recently focused on the design and synthesis of various macrocyclic polyamine [12]aneN3 compounds and their applications in artificial nucleases,DNA condensation agents,non-viral gene vectors,and chemical sensors [17, 18, 19, 20, 21].
It is well known that Cu2+ ion is the third-most abundant transition metal in human body. It exists in numerous metalloenzymes such as superoxide dismutase,cytochrome oxidase and tyrosinase which are critical forlife [22, 23]. However,overloading of Cu2+ ions in the neuronal cytoplasm can lead to Alzheimer’s disease, Wilson’s disease,and Menke’s disease [24, 25, 26]. Furthermore,ADP is an important biological phosphate which is involved in the consumption and production of ATP metabolism,such as the glycolysis,Kreb’s cycle,transporting activities of proteins,modulating ion channels and activating signaling cascades,which are the fundamental activities of life [27, 28, 29]. Thus developing new sensors to selectively and sensitively monitor both Cu2+ ions and ADP phosphate will be highly desired.
With the above consideration and to further expand the application of macrocyclic polyamines,we have designed and synthesized a [12]aneN3 modified boron dipyrromethene (BODIPY) compound 1,which can be successfully applied as highly selective and sensitive sensor for the sequential recognition of Cu2+ ions and ADP in aqueous solution and in living cells. 2. Experimental
1H NMR and 13C NMR spectra were obtained on a Bruker Avance III 400 MHz spectrometer at 25℃. Chemical shifts were referenced on residual solvents peaks. The infrared spectra were taken on a Nicolet 380 spectrometer in the range of 4000-400 cm-1. Mass spectra were acquired on a Waters Quattro Mocro spectrometer and high resolution mass spectra were acquired on a Waters LCT Premier XE spectrometer. Fluorescence spectra were measured on a Varian Cary Eclipse spectrometer. UV-vis spectra were measured on a Varian Cary 300 UV-vis spectrophotometer using solutions in 1.0 cm quartz cuvettes. Fluorescence quantum yield of samples were recorded on a Fluormax-4 spectrophotometer at room temperature with an integrating sphere system,and the machine was revaluated using standard sample before measurement. 2.1. Synthesis of 1
Sensor 1 was synthesized according to the route shown in Scheme 1. Boc-protected N-(3-azidopropyl) [12]aneN3 (5) was obtained from the azide substitution reaction with Boc-protected N-(3-bromopropyl) [12]aneN3 (4),which was prepared through the reaction of [12]aneN3 precursor [30] (2) with 1,3-dibromopropane and further hydrolysis (3) and protection by (Boc)2O. Another starting material,1,5-bis(acetynyl) BODIPY 6,was obtained according to literature method [31]. Reaction of 6 with azide 5 through copper (I) mediated click cycloaddition reaction resulted in compound 7. Target compound 1 was obtained by the deprotection of 7 under hydrogen chloride condition in ethyl acetate with yield of 70%. The detail of the syntheses can be found in the Supporting information.

|
Download:
|
| Scheme 1.The synthesis of sensor 1. | |
Compound 5: 1H NMR (400 MHz,CDCl3): δ 3.37-3.26 (m,10H),
2.47 (t,2H,J = 7.0 Hz),2.44-2.36 (m,4H),1.90-1.80 (m,2H),1.80-
1.71 (m,4H),1.71-1.64 (m,2H),1.44 (s,18H). 13C NMR (101 MHz,
CDCl3): δ 156.42,79.47,50.51,50.03,49.81,45.15,43.96,28.63,
27.52,26.64,25.60; IR (KBr,cm-1):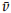 3114,2958,2096,1688,1460,
1398,1254,1158,873,804,613. ESI-MS (m/z) calcd. for
C22H40N6O4 [M+H]+: 455.3,found: 455.8.
3114,2958,2096,1688,1460,
1398,1254,1158,873,804,613. ESI-MS (m/z) calcd. for
C22H40N6O4 [M+H]+: 455.3,found: 455.8.
Compound 7: 1HNMR(400 MHz,CDCl3): δ 8.45(s,2H),7.49(dd,
5H,J = 12.7,6.6 Hz),7.30 (d,2H,J = 4.4 Hz),6.86 (d,2H,J = 4.4 Hz),
4.43 (s,4H),3.27 (dt,16H,J = 13.0,6.5 Hz),2.47 (s,4H),2.40 (s,8H),
2.11(d,4H,J = 12.2 Hz),1.82-1.75(m,5H),1.69(s,9H),1.61(s,4H),
1.37 (s,36H). 13C NMR (101 MHz,CDCl3): δ 155.37,146.89,138.45,
133.03,129.82,129.43,127.34,118.82,78.74,44.36,44.12,28.46,
28.33,27.62,27.44,21.66,13.10. IR (KBr,cm-1):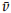 3402,2960,
2920,2851,1746,1645,1582,1554,1467,1381,1249,1129,793,
655. ESI-MS (m/z) calcd. for C63H95BF2N14O8 [M+H]+: 1225.8,
found: 1225.9.
3402,2960,
2920,2851,1746,1645,1582,1554,1467,1381,1249,1129,793,
655. ESI-MS (m/z) calcd. for C63H95BF2N14O8 [M+H]+: 1225.8,
found: 1225.9.
Compound 1: 1H NMR (400 MHz,D2O): δ 8.73 (s,2H),7.26-
6.89 (m,7H),6.45-6.20 (m,2H),4.60 (brs,4H),3.65-3.12 (m,
28H),2.45-2.31 (m,4H),2.30-1.89 (m,12H). 13C NMR (101 MHz,
D2O): δ 146.28,138.59,135.72,132.83,131.00,130.39,128.09,
126.56,119.39,50.71,48.11,47.69,42.66,41.67,24.27,20.47,
18.46,17.47,10.03. IR (KBr,cm-1):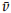 3421,2924,2852,1718,
1637,1579,1550,1467,1381,1325,1226,1141,1093,950,800,
752,721,597. HRMS (ES+) calcd. for C43H63BF2N14 (M+H)+:
825.5429,found 825.5503.
3421,2924,2852,1718,
1637,1579,1550,1467,1381,1325,1226,1141,1093,950,800,
752,721,597. HRMS (ES+) calcd. for C43H63BF2N14 (M+H)+:
825.5429,found 825.5503.
The characterization of the other new compounds and the spectra of 1H NMR,13C NMR spectroscopy and MS of all new compounds can be found in the Supporting information. 2.3. Cell culture and fluorescent image assay
HepG2 cells were cultured in DMEM supplemented with 10% FBS in a humid atmosphere containing 5% CO2 at 37℃. After 24-h incubation in the medium,for imaging studies,cells were plated in Glass Bottom Cell Culture Dish (Nest) containing 1 mL of complete DMEM and incubated at 37℃ under 5% CO2 for 24 h. Bright field and fluorescence images were taken with a Zeiss Abserver A1 inverted fluorescence microscope equipped with an EM-CCD camera (Hamamatsu) and an X-Cite 120 metal halide lamp (EXFP). Bright field and fluorescence images were obtained using an 40× objective lens. 3. Results and discussion 3.1. Fluorescent signaling of Cu2+ ions
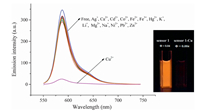
Sensor 1 displays a strong emission at 587 nm with fluorescent quantum yield of 0.041 in aqueous solution (10 μmol/L Tris-HCl, pH 7.4,25℃). The fluorescence spectra of 1 were investigated in the presence of respective fifteen metal ions including Li+,Ag+, Ca2+,Cd2+,Co2+,Cu2+,Fe3+,Fe2+,Hg2+,K+,Mg2+,Na+,Ni2+,Pb2+ and Zn2+ (Fig. 1). It can be seen that the fluorescence of 1 showed no obvious changes after the addition of metal ions except Cu2+ ion. However,the addition of Cu2+ to the solution of 1 resulted in a significant decrease (13-fold) in the fluorescence intensity at 587 nm. The quantum yieldof 1-Cu2+ complex was measured to be 0.0046. Obviously,compound 1 showed excellent selectivity for Cu2+ over other metal ions. The quench of fluorescence can be attributedtothephoto-inducedelectrontransfer(PET)mechanism caused by Cu2+ ions [32, 33].
|
Download:
|
| Fig. 1.Emission spectra of 1 (10 μmol/L) in the presence of various metal ions (5 equiv. of Ag+, Ca2+, Cd2+, Co2+, Fe2+, Fe3+, Hg2+, K+, Li+, Mg2+, Na+, Ni2+, Pb2+, Zn2+, Cu2+) in Tris-HCl buffer (1 mmol/L, pH 7.4, 25℃). λex = 540 nm. Inset: visual fluorescence changes of 1 upon addition of Cu2+ under illumination with a 365 nm UV lamp. | |
The titration profile based on the emission at 587 nm (Fig. 2a) suggested a 2:1 binding stoichiometry of Cu2+ to 1,which was further confirmed by a Job’s plot analysis (Fig. 2b).

|
Download:
|
| Fig. 2.(a) Emission spectra of 1 (10 μmol/L) in the presence of various concentrations of Cu2+ (0–40 μmol/L). (b) Job’s plot of 1 and Cu2+. The total concentration of 1 and Cu2+ was kept constant at 10 μmol/L. Condition: λex = 540 nm, λem = 587 nm, 1 mmol/L Tris–HCl buffer, pH 7.4, 25℃. | |
The binding constant for the 1-Cu2+ complex was calculated to be (1.89±0.03) × 107 L/mol based on the fluorescence titration (Fig. S1,Supporting information),indicating a strong binding ability of 1 with Cu2+. At 10 μmol/L concentration of 1,a good linear relationship between the fluorescence response and the concentration of Cu2+ (0- 20 μmol/L) was found (Fig. S2 in Supporting information). The detection limit was estimated to be 0.04 μmol/L in aqueous solution, which is significantly lower than the typical concentration of blood copper(II) (11.8-23.6 μmol/L) in normal individuals [34] and the limit of copper(II) in drinking water (GB 160 μmol/L) set by the Department of Environmental Protection of China. 3.2. Fluorescent signaling of ADP by 1-Cu2+
Fluorescent sensing of biological anions has drawn much attention in recent years [35, 36, 37]. Thus 1-Cu2+ complex was prepared in situ by mixing 1 with Cu(ClO4)2 at a 1:3 ratio in aqueous solution,and its fluorescent emission in the presence of various anions was examined. As shown in Fig. 3,a significant increase in fluorescence intensity at 587 nm was observed after the addition of ADP,the emission enhancement was 15.7-fold in the presence of 15 equiv. of ADP,while the fluorescence enhancement of ATP at the same condition was 3.6-fold. There was no obvious change of the fluorescence emission upon the addition of other organic phosphate anions and inorganic anions. This demonstrated that 1-Cu2+ complex can be successfully applied in the selective recognition of ADP. ADP also caused a color change from purple to pink in 1-Cu2+ ensemble.
The emission spectra of 1-Cu2+ complex (1 mmol/L,Tris-HCl buffer,pH 7.4) were further investigated at various concentration of ADP. As shown in Fig. S5 in Supporting information,the maximum fluorescence intensity at 587 nm started to increase when the ADP concentration was accumulated to 5 equiv. and was linear-dependent in the range of 50-150 μmol/L of ADP (R2 = 0.993),indicating that this approach was applicable for quantitative detection of ADP. No more intensity change was observed after the addition of more than 16 equiv. ADP. The limit of detection was calculated to be 0.1 μmol/L according to 3 sigma rules.

|
Download:
|
| Fig. 3.(a) Emission spectra of 1–Cu2+ (10 μmol/L) toward each of various anions (15 equiv. of ATP, ADP, AMP, GTP, GDP, GMP, CTP, CDP, CMP, UTP, UDP, UMP, PPi) in Tris–HCl buffer (1 mmol/L, pH 7.4, 25℃). λex = 540 nm. (b) The color changes of 1–Cu2+-ADP and other biological molecules under the irradiation at 365 nm. | |
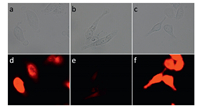
The applicability of 1 in cell imaging was then tested. It was found that HepG2 cells showed clear red fluorescence after the incubation with sensor 1 for 5 min,confirming that 1 was cellpermeable (Fig. 4a and d). Addition of Cu2+ to HepG2 cells and further incubation with compound 1 resulted in the quench of the red fluorescence emission resulted from 1 (Fig. 4b and e). With the addition of ADP to HepG2 cells,and further incubation with the 1-Cu2+ complex solution,it was found that the red fluorescence was dramatically recovered (Fig. 4c and f). These results clearly demonstrated that 1-Cu2+ complex was suitable for imaging of ADP in living cells.
|
Download:
|
| Fig. 4.Confocal fluorescence and bright field images in HepG2 cells: (a) Bright field image and (d) fluorescence image of HepG2 cells incubated with 1 (5 μmol/L) for 5 min. (b) Bright field image and (e) fluorescence image of HepG2 cells incubated with 15 μmol/L Cu(ClO4)2 for 5 min and then incubated with 1 (5 μmol/L) for 10 min. (c) Bright field image and (f) fluorescence image of HepG2 cells incubated with 100 μmol/L ADP for 10 min and then incubated with 1–Cu2+ (5 μmol/L) for 10 min. | |
In summary,a novel fluorescent sensor 1 has been prepared through the click reaction between 1,5-bis(acetynyl)BODIPY and Boc-protected N-(3-azidopropyl) [12]aneN3. The fluorescence of 1 was completely quenched upon the formation of Cu2+ complex, which provided highly selective and sensitive turn-off sensor to detect Cu2+ ions over a range of other metal ions. The detection limit was 0.04 μmol/L for Cu2+ in aqueous buffer solution. The quenched fluorescence of 1-Cu2+ complex can be selectively restored by the addition of ADP in aqueous solution and living cells. Results from this work have demonstrated that macrocyclic polyamine [12]aneN3 derivatives can be used as good receptors for fluorescent sensing applications. Sensor 1 has combined the advantages of [12]aneN3 (water soluble,strong coordinating ability) and BODIPY (longer wavelength emission and photostable),which are important for the biological applications. Further work to explore the detailed mechanism and application in metabolism process are being performed in our lab.
Acknowledgments
The authors gratefully acknowledge the financial assistances from the National Natural Science Foundation of China (Nos. 21372032 and 91227109),the Fundamental Research Funds for the Central Universities(2009SC-1),Beijing Municipal Commission of Education,the Program for Changjiang Scholars and Innovative Research Team in University.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.05.032.
| [1] | L. Fabbrizzi, A. Poggi, Anion recognition by coordinative interactions: metal–amine complexes as receptors, Chem. Soc. Rev. 42 (2013) 1681–1699. |
| [2] | J.J. Green, R. Langer, D.G. Anderson, A combinatorial polymer library approach yields insight into nonviral gene delivery, Acc. Chem. Res. 41 (2008) 749–759. |
| [3] | J.R. Morrow, T.L. Amyes, J.P. Richard, Phosphate binding energy and catalysis by small and large molecules, Acc. Chem. Res. 41 (2008) 539–548. |
| [4] | F. Liang, S. Wan, Z. Li, et al., Medical applications of macrocyclic polyamines, Curr. Med. Chem. 13 (2006) 711–727. |
| [5] | B. Le Bon, N.V. Craynest, J.M. Daoudi, et al., AMD3100 conjugates as components of targeted nonviral gene delivery systems: synthesis and in vitro transfection efficiency of cxcr4-expressing cells, Bioconjug. Chem. 15 (2004) 413–423. |
| [6] | H. Yan, Z.F. Li, Z.F. Guo, et al., Effective and reversible DNA condensation induced by bifunctional molecules containing macrocyclic polyamines and naphthyl moieties, Bioorg. Med. Chem. 20 (2012) 801–808. |
| [7] | Q.F. Zhang, W.H. Yang, W.J. Yi, et al., TACN-containing cationic lipids with ester bond: preparation and application in gene delivery, Bioorg. Med. Chem. Lett. 21 (2011) 7045–7049. |
| [8] | B. Liu, Q. Liu, J. Zhang, S. Fan, X. Yu, Transfection of nucleic acids mediated by macrocyclic polyamine-based liposomes, Prog. Chem. 25 (2013) 1237–1245. |
| [9] | H. Gao, Z. Ke, N.J. DeYonker, et al., Dinuclear Zn(II) complex catalyzed phosphodiester cleavage proceeds via a concerted mechanism: a density functional theory study, J. Am. Chem. Soc. 133 (2011) 2904–2915. |
| [10] | Z.L. Lu, C.T. Liu, A.A. Neverov, R.S. Brown, Rapid three-step cleavage of RNA and DNA model systems promoted by a dinuclear Cu(II) complex in methanol. Energetic origins of the catalytic efficacy, J. Am. Chem. Soc. 129 (2007) 11642–11652. |
| [11] | X. Sheng, X.M. Lu, J.J. Zhang, et al., Synthesis and DNA cleavage activity of artificial receptor 1,4,7-triazacyclononane containing guanidinoethyl and hydroxyethyl side arms, J. Org. Chem. 72 (2007) 1799–1802. |
| [12] | Q. Wang, H. Lönnberg, Simultaneous interaction with base and phosphate moieties modulates the phosphodiester cleavage of dinucleoside 30, 50-monophosphates by dinuclear Zn2+ complexes of di(azacrown) ligands, J. Am. Chem. Soc. 128 (2006) 10716–10728. |
| [13] | A.S. Delepine, R. Tripier, H. Handel, Cyclen-based bismacrocycles for biological anion recognition. A potentiometric and NMR study of AMP, ADP and ATP nucleotide complexation, Org. Biomol. Chem. 6 (2008) 1743–1750. |
| [14] | E. Tamanini, K. Flavin, M. Motevalli, et al., Cyclam-based “clickates”: homogeneous andheterogeneousfluorescent sensors forZn(II), Inorg.Chem.49(2010)3789–3800. |
| [15] | K. Tsuge, F. DeRosa, M.D. Lim, P.C. Ford, Intramolecular reductive nitrosylation: reaction of nitric oxide and a copper(II) complex of a cyclam derivative with pendant luminescent chromophores, J. Am. Chem. Soc. 126 (2004) 6564–6565. |
| [16] | A.T. Wrobel, T.C. Johnstone, A. Deliz Liang, S.J. Lippard, P. Rivera-Fuentes, A fast and selective near-infrared fluorescent sensor for multicolor imaging of biological nitroxyl (HNO), J. Am. Chem. Soc. 136 (2014) 4697–4705. |
| [17] | Z.F. Guo, H. Yan, Z.F. Li, Z.L. Lu, Synthesis of mono- and di-[12]aneN3 ligands and study on the catalytic cleavage of RNA model 2-hydroxypropyl-p-nitrophenyl phosphate with their metal complexes, Org. Biomol. Chem. 9 (2011) 6788–6796. |
| [18] | Z.F. Li, H.L. Chen, L.J. Zhang, Z.L. Lu, Synthesis of [12]aneN3-dipeptide conjugates as metal-free DNA nucleases, Bioorg. Med. Chem. Lett. 22 (2012) 2303–2307. |
| [19] | Z.F. Li,Z.F.Guo,H.Yan,Z.L. Lu,D.Y.Wu, Synthesesof [12]aneN3-oligopeptide conjugates as effective DNA condensation agents, Bioorg. Med. Chem. 20 (2012) 2897–2904. |
| [20] | L.J. Zhang, H.L. Chen, Z.F. Li, Z.L. Lu, R. Wang, Click-reaction generated [12]aneN3-based fluorescent sensor for Zn(II) ions, Inorg. Chem. Commun. 23 (2012) 67–69. |
| [21] | H. Yan, P. Yue, Z. Li, Z. Guo, Z. Lu, Syntheses of bifunctional molecules containing |
| [22] | aneN3 and carbazol moieties as effective DNA condensation agents, Sci. China Chem. 57 (2013) 296–306. |
| [23] | Y. Jeong, J. Yoon, Recent progress on fluorescent chemosensors for metal ions, Inorg. Chim. Acta 381 (2012) 2–14. |
| [24] | Y.W. Duan, H.Y. Tang, Y. Guo, et al., The synthesis and study of the fluorescent probe for sensing Cu2+ based on a novel coumarin Schiff-base, Chin. Chem. Lett. 25 (2014) 1082–1086. |
| [25] | J.W. Karr, V.A. Szalai, Role of aspartate-1 in Cu(II) binding to the amyloid-b peptide of Alzheimer's disease, J. Am. Chem. Soc. 129 (2007) 3796–3797. |
| [26] | B.B. Tewari, Studies on complexation in solution with a paper electrophoretic technique [the system copper(II)/cobalt(II)–methionine–penicillamine], J. Chem. Eng. Data 55 (2010) 1779–1783. |
| [27] | L. Zhang, J. Lichtmannegger, K.H. Summer, et al., Tracing copper thiomolybdathiomolybdatecomplexes in a prospective treatment for Wilson's disease, Biochemistry 48 (2009) 891–897. |
| [28] | B. Innocenti, T. Pozzan, C. Fasolato, Intracellular ADP modulates the Ca releaseactivated Ca current in a temperature- and Ca-dependent way, J. Biol. Chem. 271 (1996) 8582–8587. |
| [29] | J. Kaur, P. Singh, ATP selective acridone based fluorescent probes for monitoring of metabolic events, Chem. Commun. 47 (2011) 4472–4474. |
| [30] | A.S. Rao, D. Kim, H. Nam, et al., A turn-on two-photon fluorescent probe for ATP and ADP, Chem. Commun. 48 (2012) 3206–3208. |
| [31] | R.W. Alder, R.W. Mowlam, D.J. Vachon, G.R. Weisman, New synthetic routes to macrocyclic triamines, J. Chem. Soc. Chem. Commun. (1992) 507–508. |
| [32] | M.R. Rao, S.M. Mobin, M. Ravikanth, Boron–dipyrromethene based specific chemodosimeter for fluoride ion, Tetrahedron 66 (2010) 1728–1734. |
| [33] | N. Chopin, M. Mé debielle, O. Maury, G. Novitchi, G. Pilet, Quenching of fluorescence in bodipy-derived trifluoromethyl enaminone ligands upon coordination to copper(II), Eur. J. Inorg. Chem. 2014 (2014) 6185–6195. |
| [34] | X. Xie, Y. Qin, A dual functional near infrared fluorescent probe based on the bodipy fluorophores for selective detection of copper and aluminum ions, Sens. Actuators B 156 (2011) 213–217. |
| [35] | H.S. Jung, P.S. Kwon, J.W. Lee, et al., Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells, J. Am. Chem. Soc. 131 (2009) 2008–2012. |
| [36] | P.A. Mascaros, C. Bazzicalupi, A. Bianchi, et al., Molecular recognition of ADP over ATP in aqueous solution by a polyammonium receptor containing a pyrimidine residue, Chem. Commun. 47 (2011) 2814–2816. |
| [37] | P.J. Xie, M.L. Ye, Z.Y. Hu, et al., Determination of levels of adenosine phosphates in blood by ion chromatography, Chin. Chem. Lett. 22 (2011) 1485–1488. |
| [38] | W. Yu, J. Qiang, J. Yin, et al., Ammonium-bearing dinuclear copper(II) complex: a highly selective and sensitive colorimetric probe for pyrophosphate, Org. Lett. 16 (2014) 2220–2223. |




