The spirooxindole system is the core structure of many pharmacological agents and natural alkaloids [1, 2]. Spirooxindoles, especially those spiro-annulated with heterocycles at the 3- position,have shown good biological activities [3]. These potential properties have prompted many efforts toward the synthesis of spirooxindole-fused heterocycles and numerous impressive successes have been obtained for the synthesis of the diversely structured spirocyclic oxindoles [4, 5]. Isatin is probably one of the most widely used reagent for constructing spirooxindoles in many reactions such as 1,3-dipolar cycloaddition,Morita-Baylis-Hillman reaction and other condensation reactions [6, 7, 8]. In the past few years the multicomponent reactions based on the versatile reactivity of isatins have become the new efficient methods for the synthesis of various spirooxindoles [9, 10].
Pyranopyrazoles are fused heterocyclic compounds that exhibit a wide range of biological activities such as ngicidal,molluscicidal, anti-inflammatory activities,and act as vasodilators and hypotensive,hypoglycemic,and anticancer agents [11, 12]. Considering their clinical significance,development of efficient methods for the synthesis of dihydropyrano[2,3-c]pyrazole scaffolds have gained considerable attention from the synthetic community [13, 14, 15, 16, 17, 18, 19, 20]. In the various known synthetic methods,the four-component reaction of hydrazine hydrate,keto ester,malononitrile and aromatic aldehydes have been regarded as the most convenient method for constructing dihydropyrano[2,3-c]pyrazole skeletons. In the past years,many more green and convenient approaches for this four-component reaction have been developed [21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. When isatin was used to replace the aromatic aldehyde in the fourcomponent reaction,the corresponding spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] can be efficiently obtained [31, 32, 33, 34, 35]. Thus this four-component reaction also provided a novel method for the synthesis of spirocyclic compounds. Recently we have demonstrated that the four-component reaction of arylamine,acetylenedicarboxylate,isatin and malononitrile can afford the spiro[indoline-3,4'-pyridine] derivatives in satisfactory yields [36]. We envisioned that spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives can be easily synthesized by combining the above mentioned two four-component reactions. In continuation of our efforts toward the development of new multicomponent reactions based on the reactivity of isatin and its derivatives [37, 38, 39, 40, 41],we investigated the multicomponent reactions of hydrated hydrazine, acetylenedicarboxylate,isatin and malononitrile and developed efficient synthetic protocol for the spiro[indoline-3,4'-pyrano[2,3- c]pyrazole] derivatives. 2. Experimental 2.1. Reagents and apparatus
All reagents and solvents were commercially available with analytical grade and used as received. All evaporations of organic solvents were carried out with a rotary evaporator in conjunction with a water aspirator. Melting points were taken on a hot-plate microscope apparatus and were uncorrected. 1H NMR and 13C NMR spectra were recorded with a Bruker AV-600 instrument. IR spectra were obtained on a Bruker Tensor 27 spectrometer (KBr disc). HRMS were measured at Bruker UHR-TOF maXis spectrometer. X-ray data were collected on a Bruker Smart APEX-2 diffractometer. 2.2. General procedure for the preparation of spiro[indoline-3,4'- pyrano[2,3-c]pyrazoles] 1a-1m
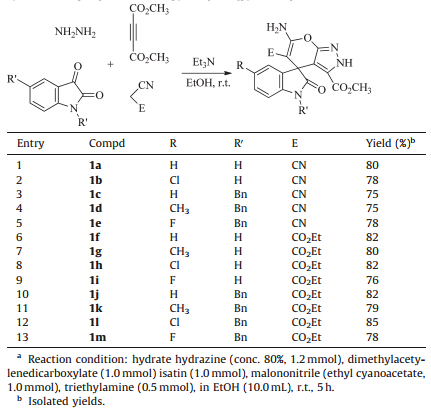
In a 50 mL round bottom flask a mixture of hydrate hydrazine (conc. 80%,1.2 mmol) and dimethyl acetylenedicarboxylate (1.0 mmol) in 10.0 mL ethanol was stirred at room temperature for about 10 min. The isatin (1.0 mmol) and malononitrile or ethyl cyanoacetate (1.0 mmol) and triethylamine (0.5 mmol) was added and the solution was stirred at room temperature for 5 h. The solvent was removed by rotatory evaporation and the residue was titrated with cold alcohol to give the pure products 1a-1m for analysis.
Methyl 6'-amino-5-chloro-5'-cyano-2-oxo-2'H-spiro[indoline- 3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate (1b): White solid,78%, mp: 248-250℃ 1H NMR (600 MHz,DMSO-d6): d 14.00 (brs,1H, NH),10.71 (s,1H,NH),7.36 (brs,2H,NH2),7.25-7.24 (m,1H,ArH), 7.11 (brs,1H,ArH),6.90-6.89 (m,1H,ArH),3.49 (s,3H,OCH3); 13C NMR (150 MHz,DMSO-d6): δ 177.3,161.2,157.7,156.0,141.1, 135.8,128.5,126.0,124.0,118.0,110.6,100.0,55.9,51.7,47.6, 30.6; IR (KBr,cm-1): υ 3429,3336,3263,3219,2950,2842,2799, 2724,2666,2196,1876,1724,1703,1645,1602,1511,1479,1447, 1402,1317,1285,1241,1215,1158,1115,1059,956,921,882, 838,819,727; MS (m/z): HRMS (ESI) Calcd. for C16H10ClN5NaO4 ([M+Na]+): 394.0314. Found: 394.0312.
Methyl 6'-amino-1-benzyl-5'-cyano-2-oxo-2'H-spiro[indoline- 3,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate (1c): White solid,75%, mp: 254-256℃ 1H NMR (600 MHz,DMSO-d6): δ 14.00 (brs,1H, NH),7.49 (d,2H,J = 7.8 Hz,ArH),7.35 (brs,2H,NH2),7.32 (t,2H, J = 7.2 Hz,ArH),7.28 (t,1H,J = 7.2 Hz,ArH),7.21 (t,1H,J = 7.8 Hz, ArH),7.06 (d,1H,J = 7.2 Hz,ArH),6.99-6.97 (m,1H,ArH),6.91 (d, 1H,J = 7.8 Hz,ArH),5.10 (d,1H,J = 15.6 Hz,CH2),4.82 (d,1H, J = 15.6 Hz,CH2),3.27 (s,3H,OCH3); 13C NMR (150 MHz, DMSO-d6): δ 176.2,161.3,157.7,156.0,142.8,136.1,132.9, 128.7,128.4,127.4,127.3,123.7,123.0,118.2,108.8,100.4,51.8, 47.1,43.4; IR (KBr,cm-1): υ 3414,3314,3256,3067,2953,2840, 2196,1910,1724,1702,1680,1637,1606,1484,1443,1393,1339, 1321,1199,1176,1060,1039,1003,959,928,909,836,811,777; MS (m/z): HRMS (ESI) Calcd. for C23H17N5NaO4 ([M+Na]+): 450.1173. Found: 450.1174.
5'-Ethyl 3'-methyl 6'-amino-5-methyl-2-oxo-2'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3',5'-dicarboxylate (1g): White solid,80%,mp: 214-216℃ 1H NMR (600 MHz,DMSO-d6): δ 13.75 (brs,1H,NH),10.24 (s,1H,NH),8.08 (brs,2H,NH2),6.89 (d, 1H,J = 7.8 Hz,ArH),6.67 (d,1H,J = 7.8 Hz,ArH),6.62 (s,1H,ArH), 3.73 (q,2H,J = 7.4 Hz,CH2),3.53 (s,3H,OCH3),2.13 (s,3H,CH3), 0.81 (t,3H,J = 7.4 Hz,CH3); 13C NMR (150 MHz,DMSO-d6): δ 179.1, 168.0,161.6,158.0,154.9,141.2,136.3,129.6,128.6,127.6,123.3, 107.9,102.7,75.3,58.9,51.4,47.4,20.5,13.0; IR (KBr,cm-1): υ 3500,3311,3201,2983,2910,2866,2743,1714,1677,1618, 1575,1544,1488,1444,1400,1324,1288,1197,1099,1044,942, 890,814,787,751; MS (m/z): HRMS (ESI) Calcd. for C19H19N4O6 ([M+H]+): 399.1299. Found: 399.1298.
5'-Ethyl 3'-methyl 6'-amino-1-benzyl-5-chloro-2-oxo-2'H-spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]-3',5'-dicarboxylate (1l): White solid,85%,mp: 246-248℃ 1H NMR (600 MHz,DMSO-d6): δ 13.89 (brs,1H,NH),8.23 (brs,2H,NH2),7.55 (d,2H,J = 7.2 Hz,ArH), 7.36 (t,2H,J = 7.8 Hz,ArH),7.30 (t,1H,J = 7.2 Hz,ArH),7.23 (d,1H, J = 8.4 Hz,ArH),7.11 (d,1H,J = 8.4 Hz,ArH),7.02 (s,1H,ArH),4.94 (d,1H,J = 15.0 Hz,CH2),4.81 (d,1H,J = 15.0 Hz,CH2),3.82-3.77 (m, 1H,CH2),3.28 (s,3H,OCH3),3.14-3.09 (m,1H,CH2),0.30 (t,3H, J = 7.2 Hz,CH3); 13C NMR (150 MHz,DMSO-d6): δ 178.8,167.8, 161.8,157.9,155.0,142.8,142.6,138.3,136.8,128.7,128.6,128.3, 127.3,127.2,124.9,122.8,109.6,109.5,101.9,74.8,59.0,51.5,50.1, 47.7,13.1; IR (KBr,cm-1): υ 3457,3382,3273,3198,3061,2954, 2789,1739,1709,1676,1612,1545,1483,1442,1402,1339,1292, 1270,1204,1167,1071,1038,918,844,810,785,748; MS (m/z): HRMS (ESI) Calcd. for C25H22ClN4O6 ([M+H]+): 509.1222. Found: 509.1218.
5'-Ethyl 3'-methyl 6'-amino-1-benzyl-5-fluoro-2-oxo-2'H-spiro [indoline-3,4'-pyrano[2,3-c]pyrazole]-3',5'-dicarboxylate (1m): White solid,78%,mp: 234-236℃ 1H NMR (600 MHz,DMSO-d6): δ 13.87 (brs,1H,NH),8.22 (brs,2H,NH2),7.55 (d,2H,J = 7.8 Hz, ArH),7.36 (t,2H,J = 7.2 Hz,ArH),7.30 (t,1H,J = 7.2 Hz,ArH),7.07- 7.05 (m,1H,ArH),7.01-6.98 (m,1H,ArH),6.89-6.87 (m,1H,ArH), 4.93 (d,1H,J = 15.0 Hz,CH2),4.80 (d,1H,J = 15.0 Hz,CH2),3.82- 3.77 (m,1H,CH2),3.25 (s,3H,OCH3),3.14-3.09 (m,1H,CH2),0.29 (t,3H,J = 7.2 Hz,CH3); 13C NMR (150 MHz,DMSO-d6): δ 177.6, 167.7,162.0,159.3,157.7,151.0,137.6,137.5,137.0,136.9,128.7, 128.3,127.5,113.3,113.1,110.6,110.4,108.2,108.1,101.5,74.6, 58.4,51.6,47.4,44.2,13.2; IR (KBr,cm-1): υ 3387,3277,3196,3060, 2932,2788,1739,1707,1678,1614,1545,1491,1447,1401,1341, 1291,1266,1172,1133,1069,1038,924,856,810,740; MS (m/z): HRMS (ESI) Calcd. for C25H22FN4O6 ([M+H]+): 493.1518. Found: 493.1516. 2.3. General procedure for the preparation of spiro[acenaphthyl-3,4'- pyrano[2,3-c]pyrazole] 2a-2c
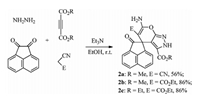
In a 50 mL round bottom flask a mixture of hydrate hydrazine (conc. 80%,1.2 mmol) and dimethyl acetylenedicarboxylate (1.0 mmol) in 10.0 mL ethanol was stirred at room temperature for about 5 min. Acenaphthenequinone (1.0 mmol),malononitrile or ethyl cyanoacetate (1.0 mmol) and triethylamine (0.5 mmol) was added and the solution was stirred at room temperature for about 24 h. The precipitates were collected by filtration and washed with cold alcohol to give the pure products 2a-2c for analysis.
Methyl 6'-amino-5'-cyano-2-oxo-2H,2'H-spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole]-3'-carboxylate (2a): White solid, 56%,mp 238-240℃ 1H NMR (600 MHz,DMSO-d6): δ 13.96 (brs, 1H,NH),8.36 (d,1H,J = 7.8 Hz,ArH),8.03 (d,1H,J = 7.2 Hz,ArH), 8.00 (d,1H,J = 8.4 Hz,ArH),7.90 (t,1H,J = 7.8 Hz,ArH),7.68 (t,1H, J = 7.8 Hz,ArH),7.38 (d,1H,J = 7.2 Hz,ArH),7.34 (brs,2H,NH),2.69 (s,3H,OCH3); 13C NMR (150 MHz,DMSO-d6): δ 203.1,161.0,157.4, 156.2,142.5,141.2,132.0,131.6,129.8,129.2,128.6,128.5,124.7, 122.0,120.5,118.2,101.2,57.3,51.4,50.9; IR (KBr,cm-1): υ 3479, 3436,3298,3175,2952,2189,1712,1641,1600,1519,1491,1446, 1398,1365,1330,1262,1214,1186,1120,1065,994,952,886, 836; MS (m/z): HRMS (ESI) Calcd. for C20H12N4NaO4 ([M+Na]+): 395.0751. Found: 395.0753.
5'-Ethyl 3'-methyl 6'-amino-2-oxo-2H,2'H-spiro[acenaphthylene-1,4'-pyrano[2,3-c]pyrazole]-3',5'-dicarboxylate (2b): White solid,86%,mp 207-209℃ 1H NMR (600 MHz,DMSO-d6): δ 13.82 (brs,1H,NH),8.23 (d,1H,J = 7.8 Hz,ArH),8.18 (brs,2H,NH),7.92 (d,1H,J = 7.2 Hz,ArH),7.87 (d,1H,J = 7.8 Hz,ArH),7.82 (t,1H, J = 7.8 Hz,ArH),7.55 (t,1H,J = 7.2 Hz,ArH),7.22 (d,1H,J = 7.2 Hz, ArH),3.30 (q,2H,J = 7.8 Hz,OCH2),2.76 (s,3H,OCH3),-0.09 (t,3H, J = 7.2 Hz,CH3); 13C NMR (150 MHz,DMSO-d6): δ 204.6,167.8, 161.8,157.7,155.0,154.9,145.5,151.3,135.0,130.3,129.4,128.5, 127.8,123.7,120.1,119.4,103.2,76.4,58.4,51.5,50.8,12.1; IR (KBr,cm-1): υ 3380,3266,2981,2951,2901,1724,1677,1623, 1569,1544,1480,1441,1401,1366,1344,1282,1189,1098,1063, 1039,988,956,923,889,838; MS (m/z): HRMS (ESI) Calcd. for C22H17N3NaO6 ([M+Na]+): 442.1010. Found: 442.1014.
Diethyl 6'-amino-2-oxo-2H,2'H-spiro[acenaphthylene-1,4'- pyrano[2,3-c]pyrazole]-3',5'-dicarboxylate (2c): White solid, 86%,mp 207-209℃ 1H NMR (600 MHz,DMSO-d6): δ 13.77 (brs,1H,NH),8.22 (d,1H,J = 8.4 Hz,ArH),8.17 (brs,2H,NH),7.92 (d,1H,J = 7.2 Hz,ArH),7.87 (d,1H,J = 8.4 Hz,ArH),7.81 (t,1H, J = 7.8 Hz,ArH),7.55 (t,1H,J = 7.8 Hz,ArH),7.22 (d,1H,J = 6.6 Hz, ArH),3.32-3.25 (m,2H,OCH2),3.23-3.17 (m,1H,OCH2),3.12-3.07 (m,1H,OCH2),0.48 (t,3H,J = 7.2 Hz,CH3),-0.09 (t,3H,J = 7.2 Hz, CH3); 13C NMR (150 MHz,DMSO-d6): δ 204.6,167.8,161.8,157.3, 155.0,145.5,141.4,135.1,130.3,129.4,128.8,128.5,128.9,123.7, 120.1,119.4,103.0,76.5,60.0,58.4,51.5,13.3,12.1; IR (KBr,cm-1): υ 3418,3304,3169,3050,2982,2959,2903,1717,1670,1611, 1570,1537,1473,1425,1402,1367,1345,1318,1196,1093,1063, 1035,992,889,858; MS (m/z): HRMS (ESI) Calcd. for C23H19N3NaO6 ([M+Na]+): 456.1166. Found: 456.1166. 3. Results and discussion
According to the reaction conditions of the previously reported the four-component reaction for the efficient synthesis of the functionalized spiro[indoline-3,4'-pyridine] derivatives [37]. A mixture of hydrated hydrazine and dimethyl acetylenedicarboxylate in ethanol was firstly stirred at room temperature for about 10 min. Then,isatin and malononitrile as well as triethylamine as the base catalyst were introduced. The reaction proceeded very smoothly at room temperature to give the expected spiro[indoline- 3,4'-pyrano[2,3-c]pyrazoles] 1a-1e in satisfactory yields (Table 1, entries 1-5). The reactions containing ethyl cyanoacetate also gave high yields of the spiro compounds 1f-1m (Table 1,entries 6-13). The structures of the prepared spiro[indoline-3,4'-pyrano[2,3- c]pyrazoles] 1a-1m were fully characterized with were characterized by IR,1H NMR,13C NMR,HRMS spectra and were further confirmed by single-crystal X-ray diffraction determination of the two compounds 1a (Fig. 1a) and 1l (Fig. 1b). The 1H NMR spectra of compounds 1a-1m usually show two singlets at about 14.00, 10.70 ppm for the two cyclic NH units and one broad singlet at about 7.30 ppm for the amino group. In the crystal structure of compound 1a,one intramolecular N-H…O hydrogen bond was formed between the amino group and carbonyl group. It should be pointed out that two similar research works about the synthesis of some spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles] by the this fourcomponent reaction have been reported very recently [32, 42].
| Table 1 Synthesis of spiro[indoline-3,40-pyrano[2,3-c]pyrazoles] 1a–1m.a |

|
Download:
|
| Fig. 1.Molecular structures of the spiro compounds 1a and 1l. | |
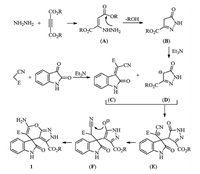
A sequential reaction mechanism is proposed for this fourcomponent reaction based on the previous reported synthetic reactions of Huisgen’s 1,4-dipoles and the spiro[indoline-3,4'- pyrano[2,3-c]pyrazole] (Scheme 1). Firstly,the addition of hydrazine to acetylenedicarboxylate forms the 2-hydrazinyl substituted but-2-enedioate (A). Secondly,the intramolecular hydrazinolysis of one ester affords a pyrazolone intermediate (B),which in turn was deprotonated by triethylamine to transform a carbanium ion (C). In the meantime,the triethylamine catalyzed condensation of isatin with malonontrile produces the isatinylidene malononitrile (D). Thirdly,a Michael addition of the carbanium ion (C) to isatinylidene malononitrile (D) gives the adduct (E). Then,the adduct (E) transforms to a emulate (F) through the keto-enol tautomerization. Finally,the intramolecular addition of enolate to cyano group results in the obtained spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] 1 with an imine-enamine tautomerization.
|
Download:
|
| Scheme 1.The proposed reaction mechanism of the four-component reaction. | |
In order to explore the potential of this protocol for spiroheterocyclic synthesis,the similar four-component reactions of hydrate hydrazine,acetylenedicarboxylate,acenaphthenequinone and malononitrile (ethyl cyanoacetate) were also investigated. The reactions can be accomplished at room temperature in 24 h to give the spiro[acenaphthyl-3,4'-pyrano[2,3-c]pyrazoles] 2a-2c in good yields (Scheme 2). The structures of spiro compounds 2a-2c were established on the spectroscopic methods and confirmed by X-ray determination of single crystal of compound 2b (Fig. 2). In the 1H NMR spectra of spiro compounds 2b and 2c,it is interesting to find that the sign of methyl group in the moiety of ester appears in very high field (-0.09 ppm),which might be caused by the induced magnetic effect of aromatic ring. From the Fig. 2,it is clear to see that the methyl group exists on the top of acenaphthenequinone.
|
Download:
|
| Scheme 2.Four-component reaction for synthesis of spiro[acenaphthyl-3,4'-pyrano[2,3-c]pyrazoles]. | |

|
Download:
|
| Fig. 2. Molecular structure of compound 2b. | |
In summary,we have developed a efficient methodology for the efficient synthesis of the spiro[indoline-3,4'-pyrano[2,3-c]pyrazoles] and spiro[acenaphthyl-3,4'-pyrano[2,3-c]pyrazoles] by the four-component reactions of hydrazine,acetylenedicarboxylate, malononitrile and isatin or acenaphthenequinone. This reaction provides a new example for the Huisgen’s 1,4-dipoles in the synthetic applications. This reaction also has the advantages of using common starting material,mild reaction conditions and operational simplicity. The potential uses of the reaction in synthetic and medicinal chemistry might be quite significant.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21172189) and the Priority Academic Program Development of Jiangsu Higher Education Institutions,China. We thanked Analysis and Test Center of Yangzhou University providing all analytical instruments.
| [1] | A.H. Abdel-Rahman, E.M. Keshk, M.A. Hanna, S.M. El-Bady, Synthesis and evaluation of some new spiro indoline-based heterocycles as potentially active antimicrobial agents, Bioorg. Med. Chem. 12 (2004) 2483–2488. |
| [2] | M.A. Koch, A. Schuffenhauer, M. Scheck, et al., Charting biologically relevant chemical space: a structural classification of natural products, Proc. Natl. Acad. Sci. U.S.A. 102 (2005) 17272–17277. |
| [3] | G.S. Singh, Z.Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks, Chem. Rev. 112 (2012) 6104–6155. |
| [4] | B.M. Trost, M.K. Brennan, Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products, Synthesis 18 (2009) 3003–3025. |
| [5] | N.R. Ball-Jones, J.J. Badillo, A.K. Franz, Strategies for the enantioselective synthesis of spirooxindoles, Org. Biomol. Chem. 10 (2012) 5165–5181. |
| [6] | L. Hong, R. Wang, Recent advances in asymmetric organocatalytic construction of 3,3'-spirocyclic oxindoles, Adv. Synth. Catal. 355 (2013) 1023–1030. |
| [7] | B. Tan, N.R. Candeias, C.F. Barbas III, Construction of bispirooxindoles containing three quaternary stereocenters in a cascade using a single multifunctional organocatalyst, Nat. Chem. 3 (2011) 473–4776. |
| [8] | F. Shi, Z.L. Tao, S.W. Luo, S.J. Tu, L.Z. Gong, Scaffold-inspired enantioselective synthesis of biologically important spiro[pyrrolidin-3,20-oxindoles] with structural diversity through catalytic isatin-derived 1,3-dipolar cycloadditions, Chem. Eur. J. 18 (2012) 6885–6894. |
| [9] | H. Deng, Y. Wei, M. Shi, Highly regio- and diastereoselective construction of spirocyclopenteneoxindoles through phosphine-catalyzed [3 + 2] annulation of Morita-Baylis-Hillman carbonates with isatylidene malononitriles, Org. Lett. 13 (2011) 3348–3351. |
| [10] | X. Li, Y. Li, F. Peng, et al., Highly enantioselective one-pot synthesis of spirocyclopentaneoxindoles containing the oxime group by organocatalyzed michael addition/ISOC/fragmentation sequence, Org. Lett. 13 (2011) 6160–6163. |
| [11] | S.C. Kuo, L.J. Huang, H. Nakamura, Studies on heterocyclic compounds. 6. Synthesis and analgesic and antiinflammatory activities of 3,4-dimethylpyrano[2,3- c]pyrazol-6-one derivatives, J. Med. Chem. 17 (1984) 539–544. |
| [12] | J.L. Wang, D. Liu, Z.J. Zheng, et al., Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells, Proc. Natl. Acad. Sci. U.S.A. 97 (2000) 7124–7129. |
| [13] | V.D. Dyachenko, E.B. Rusanov, Novel approaches to synthesis of 4-alkyl-6-amino- 5-cyano-3-methyl(propyl, phenyl)-2H,4H-pyrano[2,3-c]pyrazoles, Chem. Heterocycl. Compd. 40 (2004) 231–240. |
| [14] | A. Shaabani, E. Soleimani, A. Sarvary, A.H. Rezayan, A simple and efficient approach to the synthesis of 4H-furo[3,4-b]pyrans via a three-component reaction of isocyanides, Bioorg. Med. Chem. Lett. 18 (2008) 3968–3970. |
| [15] | A. Shaabani, A. Sarvary, A.H. Rezayan, S. Keshipour, Synthesis of fully substituted pyrano[2,3-c]pyrazole derivatives via a multicomponent reaction of isocyanides, Tetrahedron 65 (2009) 3492–3495. |
| [16] | Y.M. Litvinov, A.A. Shestopalov, L.A. Rodinovskaya, A.M. Shestopalov, New convenient four-component synthesis of 6-amino-2,4-dihydropyrano[2,3-c]pyrazol- 5-carbonitriles and one-pot synthesis of 6'-aminospiro[(3H)-indol-3,4'-pyrano[2,3-c]pyrazol]-(1H)-2-on-5'-carbonitriles, J. Comb. Chem. 11 (2009) 914–919. |
| [17] | H.M. Al-Matar, K.D. Khalil, A.Y. Adam, M.H. Elnagdi, Green one pot solvent-free synthesis of pyrano[2,3-c]-pyrazoles and pyrazolo[1,5-a]pyrimidines, Molecules 15 (2010) 6619–6629. |
| [18] | K. Kanagaraj, K. Pitchumani, Solvent-free multicomponent synthesis of pyranopyrazoles; per-6-amino-β-cyclodextrin as a remarkable catalyst and host, Tetrahedron Lett. 51 (2010) 3312–3316. |
| [19] | H. Mecadon, M.D.R. Rohman, I. Kharbangar, et al., L-Proline as an efficient catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[ 2,3-c]pyrazole-5-carbonitriles in water, Tetrahedron Lett. 52 (2011) 3228–3231. |
| [20] | S. Muramulla, C.G. Zhao, A new catalytic mode of the modularly designed organocatalysts (MDOs): enantioselective synthesis of dihydropyrano[2,3-c]pyrazoles, Tetrahedron Lett. 52 (2011) 3905–3908. |
| [21] | A.M. Shestopalov, Y.M. Emeliyanova, A.A. Shestopalov, et al., Cross-condensation of derivatives of cyanoacetic acid and carbonyl compounds. Part 1: single-stage synthesis of 10-substituted 6-amino-spiro-4-(piperidine-4')-2H,4H-pyrano[2,3-c]pyrazole-5-carbonitriles, Tetrahedron 59 (2003) 7491–7496. |
| [22] | G. Vasuki, K. Kumaravel, Rapid four-component reactions in water: synthesis of pyranopyrazoles, Tetrahedron Lett. 49 (2008) 5636–5638. |
| [23] | S. Gogoi, C.G. Zhao, Organocatalyzed enantioselective synthesis of 6-amino-5- cyanodihydropyrano[2,3-c]pyrazoles, Tetrahedron Lett. 50 (2009) 2252–2255. |
| [24] | A. Siddekha, A. Nizam, M.A. Pasha, An efficient and simple approach for the synthesis of pyranopyrazoles using imidazole (catalytic) in aqueous medium, and the vibrational spectroscopic studies on 6-amino-4-(4'-methoxyphenyl)-5-cyano- 3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole using density functional theory, Spectrochim. Acta A 81 (2011) 431–440. |
| [25] | H. Mecadon, M.R. Rohman, M. Rajbangshi, B. Myrboh, γ-Alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4- dihydropyrano[2,3-c]pyrazole-5-carbonitriles in aqueous medium, Tetrahedron Lett. 52 (2011) 2523–2525. |
| [26] | H. Mecadon, M.R. Rohman, I. Kharbangar, et al., L-Proline as an efficient catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[ 2,3-c]pyrazole-5-carbonitriles in water, Tetrahedron Lett. 52 (2011) 3228–3231. |
| [27] | S.H.S. Azzam, M.A. Pasha, Simple and efficient protocol for the synthesis of novel dihydro-1H-pyrano[2,3-c]pyrazol-6-ones via a one-pot four-component reaction, Tetrahedron Lett. 53 (2012) 6834–6837. |
| [28] | S.R. Mandha, S. Siliveri, M. Alla, et al., Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c]pyrazoles, Bioorg. Med. Chem. Lett. 22 (2012) 5272–5278. |
| [29] | A.M. Zonouz, I. Eskandari, H.R. Khavasi, A green and convenient approach for the synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole- 3-carboxylates via a one-pot, multi-component reaction in water, Tetrahedron Lett. 53 (2012) 5519–5522. |
| [30] | J. Albadi, A. Mansournezhad, Z. Derakhshandeh, CuO-CeO2 nanocomposite: a highly efficient recyclable catalyst for the multicomponent synthesis of 4Hbenzo[ b]pyran derivatives, Chin. Chem. Lett. 24 (2013) 821–824. |
| [31] | J.X. Yu, Y.B. Zhou, T.H. Shen, et al., Novel and efficient one-pot synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole]derivatives catalyzed by L-proline in aqueous medium, J. Chem. Res. 37 (2013) 365–368. |
| [32] | S. Pal, M.D. Khan, S. Karamthulla, S.J. Abbas, L.H. Choudhury, One pot fourcomponent reaction for the efficient synthesis of spiro[indoline-3,40-pyrano[2,3-c]pyrazole]-30-carboxylate derivatives, Tetrahedron Lett. 54 (2013) 5434–5440. |
| [33] | Y. Zou, Y. Hu, H. Liu, D.Q. Shi, Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro[indoline-3,4'-pyrano[2,3-c]pyrazole] derivatives, ACS Comb. Sci. 14 (2012) 38–43. |
| [34] | S. Ahadi, Z. Yasaei, A. Bazgir, A clean and one-pot synthesis of spiroindolinepyranopyrazoles, J. Heterocycl. Chem. 47 (2010) 1090–1094. |
| [35] | X.Q. Liu, X.L. Xu, X. Wang, et al., A facile and convenient way to functionalized trifluoromethylated spirocyclic[indole-3,4-pyrano[2,3-c]pyrazole] derivatives, Tetrahedron Lett. 54 (2013) 4451–4455. |
| [36] | Y. Sun, Q. Wu, L.J. Zhang, C.G. Yan, Efficient synthesis of the functionalized spiro[indoline-3,4'-pyridine] via four-component reaction, Chin. J. Chem. 30 (2012) 1548–1554. |
| [37] | Y. Sun, J. Sun, C.G. Yan, Synthesis of 10-aryl-2'-(2-oxoindolin-3-yl)spiro[indoline- 3,5'-pyrroline]-2,3'-dione via one-pot reaction of arylamines, acetone, and isatins, Tetrahedron Lett. 53 (2012) 3647–3649. |
| [38] | Y. Han, Q. Wu, J. Sun, C.G. Yan, Synthesis of the functionalized spiro[indoline-3,5'-pyrroline]-2,2'-diones via three-component reactions of arylamines, acetylenedicarboxylates, and isatins, Tetrahedron 68 (2012) 8539–8544. |
| [39] | J. Sun, Y. Sun, H. Gao, C.G. Yan, Synthesis of spiro[indoline-3,2'-quinoline] derivatives through a four-component reaction, Eur. J. Org. Chem. 10 (2012) 1976–1983. |
| [40] | L. Wu, J. Sun, C.G. Yan, Facile synthesis of spiro[indoline-3,3'-pyrrolo[1,2-a]quinolines] and spiro[indoline-3,1'-pyrrolo[2,1-a]isoquinolines] via 1,3-dipolar cycloaddition reactions of heteroaromatic ammonium salts with 3-phenacylideneoxindoles, Org. Biomol. Chem. 10 (2012) 9452–9463. |
| [41] | J. Sun, Y. Sun, H. Gong, Y.J. Xue, C.G. Yan, Synthesis of spiro[dihydropyridineoxindoles] via three-component reaction of arylamine, isatin and cyclopentane-1,3-dione, Beilstein J. Org. Chem. 9 (2013) 8–14. |
| [42] | D.M. Pore, P.B. Patil, D.S. Gaikwad, et al., Green access to novel spiro pyranopyrazole derivatives, Tetrahedron Lett. 54 (2013) 5876–5878. |