Supramolecular polymers have been prevailing since JeanMarie Lehn’s report in 1990 [1]. Unlike conventional polymers formed by the covalent bonds,the construction of supramolecular polymers is mainly based on non-covalent interactions,such as hydrogen bonds [2],metal-ligand bonds [3],host-guest recognition [4],aromatic stacking [5],and so forth. One typical branch of supramolecular polymers is constructed based on macrocycle recognition and interlocked structures. Supramolecular polymers based on macrocyclic molecules are formed generally by holding monomers or components in the big cavity of macrocycles via the highly directional non-covalent interactions. A myriad of macrocyclic components such as crown ether [6],cyclodextrin [7],cucurbituril (CB) [8],calixarene [9],pillararene [10],and their derivatives are usually employed to construct supramolecular polymers based on their recognition.
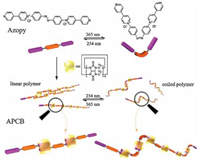
Cucurbit[n]urils (CB[n]s),a family of versatile macrocyclic hosts,can usually put the cationic guest molecules into their hydrophobic cavities through the ion dipole,hydrophobic effect and hydrogen bonding interactions between hosts and guests. The CB[n]s can include the cationic guest in their cavities with high binding constants in aqueous solution [11]. Comparing with the other members of CB[n]s family,CB[8] with bigger cavity makes it the ideal candidate being the host of the supramolecular polymers. Recently,two new rigid supramolecular polymers were constructed via the self-assembly of rigid monomers and cucurbit[8]uril (CB[8]) in water [12]. These supramolecular polymers possessed rigid backbones and further aggregated into stick-like bunched fibers. The constructed supramolecular polymers with specific functional group usually exhibit intriguing properties under external environmental stimuli [13],such as light [14],pH [15],temperature [16],and so forth. Herein,we report the construction of a simple supramolecular host-guest polymer system APCB that is stabilized by intermolecular interactions among CB[8] and 4,4'-bipyridin-1-ium (BP) units of a rigid monomer Azopy in aqueous solution,which provided a useful strategy for fabricating supramolecular polymers with significant structural rigidity. Interestingly,this rigid supramolecular polymer also exhibited intriguing photo-responsiveness owing to the photoinduced trans-cis isomerization of azobenzene [17] units of the monomers (Scheme 1),accompanying with its distinct morphology change. The linear suparmolecular polymers transformed into coiled ones in morphology under alternative light stimuli.
|
Download:
|
| Scheme 1.Schematic representation for the construction of the supramolecular polymer APCB via host–guest interaction between Azopy and CB[8], and the photoresponsive morphology changes of APCB by alternative light irradiation. | |
1H NMR spectra were measured on a Brüker AV-400 spectrometer. The electronic spray ionization (ESI) high-resolution mass spectra were tested on a HP 5958 mass spectrometer. The UV-vis absorption spectra were obtained on a Varian Cary 100 spectrometer and a Varian Cary Eclipse (1-cm quartz cell was used). The nanoparticle size was measured on NanoSight NS 300 supplied by Malvern. TEM images were recorded on JEOL JEM-1400 and JEM-2100 apparatus (droplets of the sample solution (5 × 10-5 mol/L) were applied to a perforated copper grid (400 mesh) covered with a carbon film). The UV irradiations were performed by a handheld UV lamp with an output power of 6 W.
Chloro-2,4-dinitrobenzene,4,4'-bipyridine,4,4'-diaminoazobenzene and butyl alcohol were commercially available and used without further purification. 2,4-Dinitrophenyl-4,4'-bipyridinium chloride was synthesized according to the previous report [18]. 4,4'-Bipyridine was added (6.41 g,41 mmol) to a solution of chloro-2,4-dinitrobenzene (8.31 g,41 mmol) in 60 mL EtOH and the solution was refluxed for 24 h. The dark brown solution after cooling at room temperature was added to diethyl ether (400 mL) with stirring. A golden-brown precipitate was obtained which was then filtered by suction and washed several times with diethyl ether. The solid was stored under vacuum over anhydrous CaCl2 (highly hygroscopic solid which turns to a sticky brown solid when exposed to air) to give N-(2,4-dinitrophenyl)-4,4'-bipyridinium chloride 12.08 g (33.67 mmol,82%). 1H NMR (400 MHz,DMSO-d6): δ 9.57 (d,2H,J = 6.3 Hz),9.15 (d,1H,J = 2.3 Hz),9.01 (dd,1H,J = 8.7,2.3 Hz),8.96 (d,4H,J = 4.4 Hz),8.46 (d,1H,J = 8.6 Hz),8.20 (d,2H,J = 5.2 Hz). 13C NMR (101 MHz,DMSO-d6): δ 154.99 (s),150.19 (s),149.67 (s),147.21 (s),143.56 (s),142.37 (s),138.92 (s),132.54 (s),130.73 (s),125.89 (s),123.40 (s),121.91 (s). TOF-MS: [M-Cl-]+ 323.0775,found 323.0781.
N-(2,4-dinitrophenyl)-4,4'-bipyridinium chloride (2.03 g,5.65 mmol) and 4,4'-diaminoazobenzene (0.40 g,1.88 mmol) were suspended in butyl alcohol (50 mL) in a round bottomed flask under a nitrogen atmosphere. The solution was refluxed for 3 days. After that,the reaction mixture was cooled and the solvent was removed with rotavapor,the resulting residue was subjected to column chromatography (acetonitrile/potassium nitrate solution 10:1) to give the mixture of compound Azopy and potassium nitrate. Then the compound Azopy was recrystallized from H2O. Next the mixture was cooled and filtrated to give orange solid compound Azopy (0.11 g,0.2 mmol,11%). 1H NMR (400 MHz,D2O): δ 9.29 (d,4H,J = 6.9 Hz),8.83-8.77 (m,4H),8.61 (d,4H,J = 6.9 Hz),8.25 (d,4H,J = 8.8 Hz),8.06-7.95 (m,8H). 13C NMR (101 MHz,DMSO-d6): δ 154.69-153.58 (m),153.26 (s),151.63 (s),145.90 (s),144.74 (s),141.01 (s),126.99 (s),125.78 (s),124.78 (s),122.58 (s). MASS (ESI) m/z: ([M-2Cl-]2+)/2 calcd. 246.1026,found 246.1025.
3. Results and discussionThe rigid supramolecular polymer APCB was constructed employing azobenzene derivative Azopy and CB[8] via a relatively longer binding process (stirred overnight) in aqueous solution. The two BP units from two independent Azopy monomers were entrapped together inside the cavity of CB[8] in a head-to-tail orientation [19]. The guest monomer Azopy was synthesized simply via a Zincke reaction of N-(2,4-dinitrophenyl)-4,4'-bipyridinium chloride with 4,40-diaminoazobenzene and characterized using 1H NMR,13C NMR and MS spectra (Figs. S10-S12 in,Supporting information). The formation of the APCB between Azopy and CB[8] was explicitly stated by the 1H NMR spectroscopy (Fig. 1) and 1H NOESY spectroscopic experiments in D2O (Fig. S4 in Supporting information). Diffusion-ordered NMR spectroscopy (DOSY) was further utilized to evaluate the polymerization efficiency of the supramolecular polymerization system (Figs. S2,S3 in Supporting information). The average diffusion coefficients of Azopy and APCB were measured to be 3.16 × 10-10 m2/s and 1.79 × 10-10 m2/s,respectively. The decrease of diffusion coefficient of APCB implies that bulky polymeric species are formed. As we can see from Fig. 1,the NMR signal of Ha,Hb,Hc, Hd,He and Hf shifted upfield by 0.49 ppm,0.70 ppm,0.56 ppm,0.10 ppm,0.40 ppm,0.25 ppm,respectively,owing to the inclusion interaction of CB[8]. Besides,the envisaged inclusion pattern of Azopy and CB[8] was specifically observed from 2D 1H NMR NOESY. The NOE correlations between Ha and Hc,Hb and Hd were observed. This fact also demonstrated that two 4,4'-bipyridin-1- ium units were included inside the cavity of CB[8] in a manner of head-to-tail orientation perfectly. Also,the 1:1 binding stoichiometry was clearly confirmed by Job’s plot (Fig. S1 in Supporting information) investigation.

|
Download:
|
| Fig. 1.Partial 1H NMR spectra of (a) Azopy, (b) APCB (Azopy: CB[8] = 1:1) in D2O at 25 ℃, both concentrations were 1.0 mmol/L. | |
The UV-vis absorption spectra of Azopy and supramolecular polymer APCB were also employed to further illustrate the interaction between CB[8] and Azopy. As seen in Fig. S5 (Supporting information),under the same conditions,the absorption peaks at 268 nm and 335 nm corresponding to Azopy showed red-shifts to 272 nm and 347 nm,respectively,as a result of the formation of APCB.
It is known to all that azobenzene derivatives can undergo a reversible trans-cis photoisomerization of their representative N5 5N double bond under specific light irradiation. Similarly,the trans-Azopy would undergo a change in terms of configuration when exposed to the light of 365 nm. This phenomenon can be clearly confirmed from the UV-vis absorbance changes (Fig. 2a). When irradiated with light of 365 nm,the absorption peak corresponding to trans-Azopy at 335 nm decreased,and then reached the photostationary state rapidly. After the initial Azopy system has photoisomerized,the equilibrium can be reversed by irradiation at 254 nm. In general,the interval of any process was about 60 s between the two photostationary states. The Azopy has a low efficiency of isomerization owing to the action of the electron-withdrawing groups [20]. While no apparent variation of the absorption peak corresponding to supramolecular polymer APCB at 347 nm was observed when irradiated using the light of 365 nm under the same condition and the same concentration as Azopy. In this case,the APCB was irradiated with the light of 254 nm. Upon irradiation by 254 nm light for 2 min,the absorption peak corresponding to 347 nm decreased immediately,the reason of which might be that the rigidity of the polymer APCB made it difficult to isomerize thus needing light irradiation of higher energy at 254 nm. This downward trend continued until 2 h plus 30 min and then reached the photostationary state by prolonged light irradiation. Meanwhile,the color of the APCB solution in the cuvette gradually turned yellow and deepened owing to the gradual rise of the absorption peak in the visible range (Fig. 2b). It seems during this entire process,the variation range of APCB is more than the scope of Azopy. However,comparing with the monomer Azopy,APCB underwent a slower photoisomerized process with a longer time under the same experimental conditions. The inverse process could proceed to some extent by irradiation of 365 nm light. The photo-responsiveness process of the supramolecular polymer APCB was reversible. However,its rigidity and further aggregation made it isomerize needing light irradiation of higher energy at 254 nm and then recover taking much longer time (about 9.5 h,about 67% of the initial absorption intensity when reaching the photostationary state) than the monomer Azopy. More photo-responsive reversibility cycles were not investigated. And the absorption peak at 347 nm recovered about 67% of the initial APCB solution when it reached the photostationary state.

|
Download:
|
| Fig. 2.UV–vis absorbance spectra of (a) Azopy irradiated by prolonged light source of 365 nm and then by 254 nm, (b) APCB irradiated by light source of 254 nm and then by 365 nm. Both concentrations were 1.5 × 10-5 mol/L in aqueous solution. | |
Based on the above results,it can be summarized that the supramolecular polymer APCB was photoisomerized upon irradiation by 254 nm light rather than the light of 365 nm and their intervals of the two photostationary states were longer than that of Azopy. From the following transmission electron microscopy (TEM) images and nanoparticle size data we could learn that the size of APCB was much larger than Azopy that the wavelength with higher energy is needed to engender its isomerization completely. Similarly,the APCB needed more time to make their Azopy units reach the photostationary states owing to its large dimension.
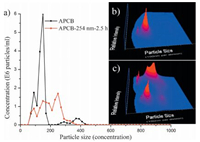
The nanoparticle tracking analysis (NTA,the NanoSight instruments) was conducted to study the size of APCB in aqueous solution. As shown in Fig. 3,the sizes of APCB mostly ranged from 70 to 200 nm (the portion from 200 to 450 nm was of a small concentration). Successively irradiating the same solution by 254 nm light for 2.5 h resulted in the disappearance of the peak larger than 400 nm. The resulted size distribution of the APCB ranged from 70 to 390 nm evenly. Nevertheless,the difference in scattering intensities displayed by the 3D graphs proved to be very useful to confirm the presence of different populations of similar sizes,such as in the sample of APCB irradiated by 254 nm for 2.5 h. Fig. 3b showed only one signal of scatting intensity corresponding to trans-APCB,while two distinct signals of scattering intensities could be found owing to the existence of two different nanoparticles corresponding to both trans-APCB and the cis-APCB.
|
Download:
|
| Fig. 3. (a) The size profiles of APCB and APCB irradiated by 254 nm for 2.5 h; and three-dimensional plot (relative intensity/particle size) of (b) APCB and (c) APCB irradiated by 254 nm for 2.5 h, evaluated by Nanosight technology. Both concentrations were 1.5 × 10-5 mol/L in aqueous solution. | |
The supramolecular polymers APCB was photo-responsive,with the morphology change after light irradiation at 254 nm. As shown in the TEM images (Fig. 4a),the morphology of APCB with length of hundreds of nanometres was observed in both linear and rigid form. It was similar with the previous report that the combined pattern between Azopy and CB[8] was head-to-tail orientation rather than the [2 + 2] head-to-head dimers [18]. The size of APCB measured by TEM is much bigger than that by NTA. The reason of this might be attributed that the TEM samples were prepared by a drying method in which surface tension of a volatile solvent during drying might cause further aggregation of the small-sized polymers,while NTA data were measured in the solution in which the polymers could be dispersed uniformly. Moreover,the higher concentration of APCB solution used to prepare TEM sample might lead to further aggregation to engender large-sized morphology of supramolecular polymers. The thicker supramolecular polymers were formed by the aggregation of individual ones [12]. Another TEM sample was prepared using the same solution of APCB after irradiation by 254 nm light for 2.5 h and observed. As seen in Fig. 4b,the original large size of the linear and rigid APCB morphology disappeared with the emergence of smaller curved and coiled ones. The change was attributed to the trans-cis photoisomerization of Azopy units in APCB,which turned the linearity of the supramolecular polymers into curved and coiled ones.

|
Download:
|
| Fig. 4.TEM images of (a) Azopy + CB[8] (1:1, 5 × 10-5 mol/L), (b) (a) after irradiation by 254 nm for 2.5 h, (c) Azopy (1 × 10-4 mol/L), (d) CB[8] (1 × 10-4 mol/L). | |
In summary,we have successfully constructed a novel linear and rigid supramolecular polymers APCB based on host-guest interaction between CB[8] and the rigid azobenzene-containing Azopy monomer in aqueous solution,and investigated its photoisomerized property and morphology transition. APCB showed a good photoisomerized property and distinct morphology change under alternative UV/vis light stimuli in aqueous solution. This new concept and strategy to make use of azobenzene derivatives by introducing them into rigid supramolecular polymers are expected to develop novel smart photo-responsive materials with potential applications.
Acknowledgments
This work was financially supported by NNSFC (Nos. 21190033,21272072,21476075 and 21372076),the National Basic Research 973 Program (No. 2011CB808400),the Shanghai Pujiang Program (No. 13PJD011) and the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01.032.
| [1] | C. Fouquey, J.M. Lehn, A.M. Levelut, Molecular recognition directed self-assembly of supramolecular liquid crystalline polymers from complementary chiral components, Adv. Mater. 2 (1990) 254–257. |
| [2] | (a) R.P. Sijbesma, F.H. Beijer, E. Meijer, et al., Reversible polymers formed from self-complementary monomers using quadruple hydrogen bonding, Science 278 (1997) 1601–1604; (b) M.E. Belowich, C. Valente, J.F. Stoddart, et al., Positive cooperativity in the template-directed synthesis of monodisperse macromolecules, J. Am. Chem. Soc. 134 (2012) 5243–5261; (c) Z.M. Shi, C.F. Wu, Z.T. Li, et al., Foldamer-based chiral supramolecular alternate block copolymers tunedbyion-pair binding,Chem. Commun. 49(2013)2673–2675; (d) T. Park, S.C. Zimmerman, A supramolecular multi-block copolymer with a high propensity for alternation, J. Am. Chem. Soc. 128 (2006) 13986–13987; (e) X. Fu, Q. Zhang, D.H. Qu, et al., A fluorescent hyperbranched supramolecular polymer based on triple hydrogen bonding interactions, Polym. Chem. 5 (2014) 6662–6666; (f) A. Noro, M. Hayashi, Y. Matsushita, Design and properties of supramolecular polymer gels, Soft Matter 8 (2012) 6416–6429. |
| [3] | (a) N.N. Adarsh, P. Dastidar, Coordination polymers: what has been achieved in going from innocent 4,4'-bipyridine to bis-pyridyl ligands having a non-innocent backbone? Chem. Soc. Rev. 41 (2012) 3039–3060; (b) Z. Li, A.M. Jamieson, S.J. Rowan, Stimuli-responsive europium-containing metallo-supramolecular polymers, J. Mater. Chem. 20 (2010) 145–151; (c) Y. Liu, Z. Wang, X. Zhang, et al., Cucurbit[8]uril-based supramolecular polymers: promoting supramolecular polymerization by metal-coordination, Chem. Commun. 49 (2013) 5766–5768; (d) S. Chen, X. Zhao, Z.T. Li, et al., Highly stable chiral (A)6–B supramolecular copolymers: a multivalency-based self-assembly process, J. Am. Chem. Soc. 133 (2011) 11124–11127. |
| [4] | (a) X. Ma, H. Tian, Stimuli-responsive supramolecular polymers in aqueous solution, Acc. Chem. Res. 47 (2014) 1971–1981; (b) X. Ma, H. Tian, Bright functional rotaxanes, Chem. Soc. Rev. 39 (2010) 70–80; (c) Q. Zhang, X. Yao, X. Ma, et al., Multistate self-assembled micro-morphology transitions controlled by host–guest interactions, Chem. Commun. 50 (2014) 1567–1569; (d) X. Ji, Y. Yao, F. Huang, et al., A supramolecular cross-linked conjugated polymer network for multiple fluorescent sensing, J. Am. Chem. Soc. 135 (2013) 74–77; (e) X. Yan, D. Xu, F. Huang, et al., A multiresponsive, shape-persistent, and elastic supramolecular polymer network gel constructed by orthogonal self-assembly,, Adv. Mater. 24 (2012) 362–369; (f) Y. Liu, Y. Yu, X. Zhang, et al., Water-soluble supramolecular polymerization driven by multiple host-stabilized charge-transfer interactions, Angew. Chem. Int. Ed. 49 (2010) 6576–6579. |
| [5] | (a) S. Burattini, B.W. Greenland, H. Colquhoun, et al., A supramolecular polymer based on tweezer-type π–π stacking interactions: molecular design for healability and enhanced toughness, Chem. Mater. 23 (2011) 6–8; (b) Y. Liu, Z. Wang, X. Zhang, et al., Host-enhanced π–π interaction for watersoluble supramolecular polymerization, Chem. Eur. J. 17 (2011) 9930–9935. |
| [6] | (a) B. Zheng, F. Wang, F. Huang, et al., Supramolecular polymers constructed by crown ether-based molecular recognition, Chem. Soc. Rev. 41 (2012) 1621–1636; (b) Z. Zhang, Y. Luo, F. Huang, et al., Formation of linear supramolecular polymers that is driven by C–H·π interactions in solution and in the solid state, Angew. Chem. Int. Ed. 50 (2011) 1397–1401; (c) S. Dong, Y. Luo, F. Huang, et al., A dual-responsive supramolecular polymer gel formed by crown ether based molecular recognition, Angew. Chem. Int. Ed. 50 (2011) 1905–1909; (d) F. Wang, C.Y. Han, F. Huang, et al., Self-sorting organization of two heteroditopicmonomers to supramolecular alternating copolymers, J. Am. Chem. Soc. 130 (2008) 11254–11255; (e) H. Wang, Z.J. Zhang, Y. Liu, et al., Synthesis of a bistable [3]rotaxane and its pH-controlled intramolecular charge-transfer behavior, Chin. Chem. Lett. 24 (2013) 563–567; (f) H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545–552. |
| [7] | (a) L.L. Zhu, X. Li, H. Tian, et al., Photolockable ratiometric viscosity sensitivity of cyclodextrin polypseudorotaxane with light-active rotor graft, Langmuir 25 (2009) 3482–3486; (b) H. Chen, X. Ma, S. Wu, H. Tian, A rapidly self-healing supramolecular polymer hydrogel with photostimulated room-temperature phosphorescence responsiveness, Angew. Chem. Int. Ed. 53 (2014) 14149–14152;(c) Q. Zhang, D.H. Qu, H. Tian, et al., A dual-modality photoswitchable supramolecular polymer, Langmuir 29 (2013) 5345–5350; (d) L. Qin, P.F. Duan, M.H. Liu, Interfacial assembly and host–guest interaction of anthracene-conjugated l-glutamate dendron with cyclodextrin at the air/water interface, Chin. Chem. Lett. 25 (2014) 487–490; (e) Y.W. Yang, Y.L. Sun, N. Song, Switchable host–guest systems on surfaces, Acc. Chem. Res. 47 (2014) 1950–1960; (f) X. Ma, Q. Wang, H. Tian, Photo-driven molecular shuttles, Prog. Chem. 21 (2009) 106–115. |
| [8] | (a) Z. Huang, L. Yang, Y. Liu, X. Zhang, Supramolecular polymerization promoted and controlled through self-sorting, Angew. Chem. Int. Ed. 53 (2014) 5351–5355; (b) Z.J. Zhang, H.Y. Zhang, Y. Liu, et al., Interconversion between [5]pseudorotaxane and [3]pseudorotaxane by pasting/detaching two axle molecules, J. Org. Chem. 76 (2011) 8270–8276; (c) H. Yang, Z. Ma, X. Zhang, et al., Fabricating covalently attached hyperbranched polymers by combining photochemistry with supramolecular polymerization, Polym. Chem. 5 (2014) 1471–1476; (d) C. Gao, S. Silvi, X. Ma, et al., Chiral supramolecular switches based on (R)- binaphthalene-bipyridinium guests and cucurbituril hosts, Chem. Eur. J. 18 (2012) 16911–16921; (e) C. Gao, S. Silvi, X. Ma, et al., Reversible modulation of helicity in a binaphthyl– bipyridinium species and its cucurbit[8]uril complexes, Chem. Commun. 48 (2012) 7577–7579. |
| [9] | (a) D.S. Guo, Y. Liu, Calixarene-based supramolecular polymerization in solution, Chem. Soc. Rev. 41 (2012) 5907–5921; (b) R. Sun, Q. Zhang, X. Ma, et al., Novel supramolecular CT polymer employing disparate pseudorotaxanes as relevant monomers, Polymer 54 (2013) 2506–2510; (c) B.T. Zhao, X.M. Zhu,W.M. Zhu, et al., Novel clicked tetrathiafulvalene-calix[4]- arene assemblies: synthesis and intermolecular electron transfer toward p-chloranil, Chin. Chem. Lett. 24 (2013) 573–577. |
| [10] | (a) M. Xue, Y. Yang, F. Huang, et al., Pillararenes, a new class of macrocycles for supramolecular chemistry, Acc. Chem. Res. 45 (2012) 1294–1308; (b) X.Y. Hu, X. Wu, L. Wang, et al., Pillar[5]arene-based supramolecular polypseudorotaxane polymer networks constructed by orthogonal self-assembly, Polym. Chem. 4 (2013) 4292–4297; (c) N. Song, Y.W. Yang, Applications of pillarenes, an emerging class of synthetic macrocycles, Sci. China Chem. 57 (2014) 1185–1198. |
| [11] | (a) J. Lagona, P. Mukhopadhyay, L. Isaacs, et al., The cucurbit[n]uril family, Angew. Chem. Int. Ed. 44 (2005) 4844–4870; (b) J.W. Lee, S. Samal, K. Kim, et al., Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry, Acc. Chem. Res. 36 (2003) 621–630; (c) F. Sakai, Z.W. Ji, G.S. Chen, et al., A novel supramolecular graft copolymer via cucurbit[8]uril-based complexation and its self-assembly, Chin. Chem. Lett. 24 (2013) 568–572. |
| [12] | (a) F. Lin, T.G. Zhan, X. Zhao, et al., The construction of rigid supramolecular polymers in water through the self-assembly of rod-like monomers and cucurbit[ 8]uril, Chem. Commun. 50 (2014) 7982–7985; (b) L. Zhang, T.Y. Zhou, Z.T. Li, et al., A two-dimensional single-layer supramolecular organic framework that is driven by viologen radical cation dimerization and further promoted by cucurbit[8]uril, Polym. Chem. 5 (2014) 4715–4721; (c) C. Zhou, J. Tian, Z.T. Li, et al., A three-dimensional cross-linking supramolecular polymer stabilized by the cooperative dimerization of the viologen radical cation, Polym. Chem. 5 (2014) 341–345. |
| [13] | (a) X. Yan, F. Wang, F. Huang, et al., Stimuli-responsive supramolecular polymeric materials, Chem. Soc. Rev. 41 (2012) 6042–6065; (b) L. Sambe, V. de La Rosa, P. Woisel, Programmable polymer-based supramolecular temperature sensor with a memory function, Angew. Chem. Int. Ed. 53 (2014) 5044–5048. |
| [14] | (a) J. del Barrio, P.N. Horton, O.A. Scherman, et al., Photocontrol over cucurbit[ 8]uril complexes: stoichiometry and supramolecular polymers, J. Am. Chem. Soc. 135 (2013) 11760–11763; (b) Q. Zhang, X. Ma, H. Tian, et al., Sol–gel conversion based on photoswitching between noncovalently and covalently linked netlike supramolecular polymers, Chem. Commun. 49 (2013) 9800–9802; (c) R. Sun, X. Ma, H. Tian, et al., Light-driven linear helical supramolecular polymer formed by molecular-recognition-directed self-assembly of bis(p-sulfonatocalix[ 4]arene) and pseudorotaxane, J. Am. Chem. Soc. 135 (2013) 5990–5993; (d) X. Yao, T. Li, S. Wang, X. Ma, H. Tian, A photochromic supramolecular polymer based on bis-p-sulfonatocalix[4]arene recognition in aqueous solution, Chem. Commun. 50 (2014) 7166–7168; (e) Z. Yan, J.F. Xu, P.J. Stang, et al., Photoinduced transformations of stiffstilbenebase discrete metallacycles to metallosupramolecular polymers, Proc. Natl. Acad. Sci. U. S. A. 111 (2014) 8717–8722. |
| [15] | (a) S. Dong, B. Zheng, F. Huang, et al., A crown ether appended super gelator with multiple stimulus responsiveness, Adv. Mater. 24 (2012) 3191–3195; (b) X. Ji, S. Dong, F. Huang, et al., A novel diblock copolymer with a supramolecular polymer block and a traditional polymer block: preparation, controllable selfassembly in water, and application in controlled release, Adv. Mater. 25 (2013) 5725–5729; (c) X. Yao, X. Ma, H. Tian, Aggregation-induced emission encoding supramolecular polymers based on controllable sulfonatocalixarene recognition in aqueous solution, J. Mater. Chem. C 2 (2014) 5155–5160; (d) X. Ma, R. Sun, H. Tian, et al., Novel electrochemical and pH stimulus-responsive supramolecular polymer with disparate pseudorotaxanes as relevant unimers, Polym. Chem. 2 (2011) 1068–1070. |
| [16] | M. Zhang, D. Xu, F. Huang, et al., Self-healing supramolecular gels formed by crown ether based host–guest interactions, Angew. Chem. Int. Ed. 51 (2012) 7011–7015. |
| [17] | (a) X. Ma, Q. Wang, H. Tian, et al., A light-driven pseudo[4]rotaxane encoded by induced circular dichroism in a hydrogel, Adv. Funct. Mater. 17 (2007) 1409– 1411; (b) X. Ma, D. Qu, H. Tian, et al., A light-driven [1]rotaxane via self-complementary and suzuki-coupling capping, Chem. Commun. 14 (2007) 1409; (c) X. Ma, J. Cao, H. Tian, et al., Photocontrolled reversible room temperature phosphorescence (RTP) encoding b-cyclodextrin pseudorotaxane, Chem. Commun. 47 (2011) 3559–3561; (d) J. Cao, X. Ma, H. Tian, et al., INHIBIT logic operations based on light-driven bcyclodextrin pseudo[1]rotaxane with room temperature phosphorescence addresses, Chem. Commun. 50 (2014) 3224–3226. |
| [18] | (a) I. Yamaguchi, H. Higashi, S. Shigesue, et al., N-Arylated pyridinium salts having reactive groups, Tetrahedron Lett. 48 (2007) 7778–7781; (b) R. Papadakis, I. Deligkiozi, A. Tsolomitis, Synthesis and characterization of a group of new medium responsive nonsymmetric viologens, chromotropism and structural effects, Dyes Pigm. 95 (2012) 478–484. |
| [19] | (a) K.D. Zhang, X. Zhao, Z.T. Li, Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water, J. Am. Chem. Soc. 135 (2013) 17913–17918; (b) Y.L. Liu, H. Yang, X. Zhang, Cucurbit[8]uril-based supramolecular polymers, Chem. Asian J. 8 (2013) 1626–1632. |
| [20] | (a) I. Deligkiozi, R. Papadakis, A. Tsolomitis, Synthesis, characterisation and photoswitchability of a new [2]rotaxane of α-cyclodextrin with a diazobenzene containing π-conjugated molecular dumbbell, Supramol. Chem. 24 (2012) 333–343; (b) I. Deligkiozi, R. Papadakis, A. Tsolomitis, Photoconductive properties of a π-conjugated α-cyclodextrin containing [2]rotaxane and its corresponding molecular dumbbell, PhysChemChemPhys 15 (2013) 3497–3503. |




