Stimuli-responsive nanoparticles show good potential in the fields of controllable drug and gene delivery [1, 2, 3]. Among various smart nanoparticles employing the responsiveness toward external stimuli such as pH [4, 5],light [6, 7],temperature [8],enzymes [9, 10] and redox [11],redox-responsive nanoparticles have drawn wide attention owing to their controllable encapsulation and delivery of biological/medicinal substrates in physiological environments. Selenium is a semimetallic chemical element [12] and can be incorporated in proteins to make selenoproteins,which prevent cellular damage from free radicals [13]. Besides its biological functions,selenium also possesses unique chemical properties such as the weaker electronegativity of selenium and the bigger radius than sulfur [14],which makes the selenium compounds easier to be oxidized or reduced. In addition,with a lower bond energy (Se-Se 172 kJ/mol) than the disulfide (S-S 240 kJ/mol),the diselenide is regarded as a good candidate to build the redox-responsive system [15]. Xu,Zhang and co-workers constructed several diselenide-containing polymers by condensing organic diselenocyanates or diselenosulfates with an acid or base initiator and investigated their self-assembly behavior as well as applications as physiological condition-responsive drug delivery vehicles [16, 17, 18, 19, 20]. However,selenium-containing nanoparticles are rarely reported due to the relatively difficult preparation [16]. Recently,we developed an easy way to fabricate nanoparticles based on the complexation of p-sulfonatocalixarenes [21, 22, 23, 24],which could decrease the critical aggregation concentration (CAC),enhance the aggregate stability,and regulate the degree of order in the aggregates. Using the similar strategy,a number of supramolecular assemblies were successfully constructed through the induced aggregation of different macrocycles including cyclodextrin [25, 26],pillararene [27, 28, 29] and cucurbituril [30, 31],etc. Herein,we wish to report another type of induced aggregation,i.e.,the guest-induced aggregation of amphiphilic calixarene,where p-sulfonatocalix[4]arene tetrahexyl ether (SC4AH) and selenocystamine dihydrochloride (Se-Cys) (Scheme 1) were selected as host and guest,respectively. It is our special interest to investigate the possibility of guest Se-Cys to induce the aggregation of SC4A-H and the responsiveness of the resultant assembly to redox stimuli.

|
Download:
|
| Scheme 1. Structures of SC4A-H, Se-Cys and Nile Red. | |
Selenocystamine dihydrochloride (Se-Cys) was purchased from Sigma. Nile Red was purchased from J&K Scientific. GSH was purchased from Aladdin. All of chemicals were used without further purification. SC4A-H was synthesized according to the reported procedure [32]. A thermostated and fully computeroperated isothermal calorimetry (VP-ITC) instrument,purchased from Microcal Inc.,Northampton,MA,was used for all microcalorimetric experiments. All microcalorimetric titrations were performed in aqueous solution at atmospheric pressure and 298.15 K. Each solution was de-gassed and thermostated by a ThermoVac accessory before the titration experiment. Twentyeight successive injections were made for the titration experiment. A constant volume (10 mL/injection) of SC4A-H solution in a 0.250 mL syringe was injected into the reaction cell (1.4227 mL) charged with redistilled water. High-resolution TEM images were acquired using a high-resolution TEM (Tecnai G2 F20 microscope,FEI) equipped with a CCD camera (Orius 832,Gatan) operating at an accelerating voltage of 200 kV. The sample for TEM measurements was prepared by dropping the solution onto a copper grid. The grid was then air-dried. The sample solution for DLS measurements was prepared by filtering the solution through a 450 nm Millipore filter into a clean scintillation vial. The samples were examined on a laser light scattering spectrometer (BI-200SM) equipped with a digital correlator (TurboCorr) at 636 nm at a scattering angle of 90°. Steady-state fluorescence spectra were recorded in a conventional quartz cell (light path 10 mm) on a Varian Cary Eclipse equipped with a Varian Cary single-cell peltier accessory to control temperature. λex = 550 nm; bandwidth (ex),10 nm; bandwidth (em),5 nm.
3. Results and discussionBefore studying the aggregation of SC4A-H induced by Se-Cys,we investigated the self-aggregation behavior of SC4A-H by means of isothermal titration microcalorimetry (ITC) experiment and fluorescence spectroscopy (using Nile Red as a probe) [33]. As seen in Fig. 1,the fluorescence intensity of Nile Red gradually enhanced with the addition of SC4A-H. Generally,the hydrophobic Nile Red tends to fluorescence in the hydrophobic microenvironment,such as the interior of SC4A-H micelles. The significantly enhanced fluorescence of Nile Red above 0.33 mmol/L may indicate the formation of SC4A-H micelles in aqueous solution. The critical aggregation concentration (CAC) of SC4A-H obtained from the fluorescence spectroscopy was 0.33 mmol/L,which is basically consistent with that (0.4 mmol/L ITC. According to a reported method [32],the aggregation degree of SC4A-H at CAC was calculated to be 18 at 298.15 K.

|
Download:
|
| Fig. 1. (a) Dependence of the fluorescence intensity of Nile Red at 650 nm in aqueous solution of SC4A-H on SC4A-H concentration (25 ℃, pH 7.0). (b) Titration of 25 aliquots (10 μL) of 4.92 × 103 μmol/L solution of SC4A-H into pure water at 25 ℃. Observed reaction enthalpy (ΔHobs) versus the total SC4A-H concentration in the reaction cell. The red line represents the first derivative of ΔHobs against the concentration of SC4A-H. | |
Subsequently,the aggregation behavior of SC4A-H in the presence of Se-Cys was investigated. Fig. 2 displays the fluorescence spectra of Nile Red in the SC4A-H/Se-Cys system,and the CAC value of SC4A-H in the presence of Se-Cys (50 mmol/L) was measured to be 0.035 mmol/L,which is nearly 10 times lower than that of free SC4A-H. This result demonstrates that the complexation with Se-Cys can efficiently decrease the CAC value of SC4A-H.

|
Download:
|
| Fig. 2. (a) Fluorescence spectra of Nile Red at different SC4A-H concentrations in the presence of Se-Cys (50 μmol/L) and (b) dependence of the fluorescence intensity of Nile Red at 652 nm on SC4A-H concentration (25 ℃, pH 7.0). | |
It is also important to determine the preferable mixing ratio between SC4A-H and Se-Cys for constructing a robust supramolecular assembly. Fig. 3 shows the fluorescence spectra of Nile Red with increasing the Se-Cys concentration in the presence of SC4AH (0.1 mmol/L) and the plot of fluorescence intensity at 650 nm as a function of the concentration of Se-Cys. As seen from Fig. 3,the fluorescence intensity at 650 nm firstly increased and then gradually decreased thereafter to a quasi-plateau with increasing the concentration of Se-Cys,and the maximum was reached at the concentration of Se-Cys as 0.03 mmol/L. The increase of fluorescence intensity indicated that a supramolecular assembly was formed between SC4A-H and Se-Cys. Then,the addition of an excess amount of SC4A-H led to the formation of a simple inclusion complex,resulting in the decrease of fluorescence intensity. According to Fig. 3b,the preferable mixing ratio between Se-Cys and SC4A-H was measured to be 3:10.

|
Download:
|
| Fig. 3. (a) Fluorescence spectra of Nile Red (80 μmol/L) in the presence of SC4A-H and Se-Cys ([SC4A-H] = 0.1 mmol/L, [Se-Cys] = 0.004 mol/L to 240 μmol/L. (b) Dependence of fluorescence intensity of Nile Red at 650 nm on the concentration of Se-Cys (25 ℃, pH 7.0). | |
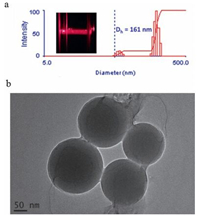
Dynamic light scattering (DLS) and transmission electron microscope (TEM) gave the information about the size and morphology of assembly. In the DLS experiment,the SC4A-H/SeCys assembly showed a hydrodynamic diameter (Dh) of 161 nm (Fig. 4a),while the free SC4A-H shows no appreciable signals at the same concentration. In addition,the clear Tyndall effect of SC4A-H/Se-Cys solution also indicates the formation of large aggregates (Fig. 4a). Significantly,TEM image showed a number of spherical particles with an average diameter of 150 nm,which fits well with the DLS results (Fig. 4b). 1H NMR measurement was used to determine the structure of nanoparticles. As shown in Fig. 5,the proton signals of methylene groups in Se-Cys exhibited certain upfield shifts. This indicates that the methylene groups in Se-Cys penetrate into the calixarene cavity,leading to the ring current effect of the aromatic nuclei of calixarene. Therefore,we deduce that SC4A-H/Se-Cys complexes form multi-lamellar spheres.
|
Download:
|
| Fig. 4. (a) Tyndall effect and DLS data of SC4A-H/Se-Cys assembly. [SC4AH] = 80 μmol/L; [Se-Cys] = 30 μmol/L. (b) TEM image of SC4A-H/Se-Cys assembly. | |

|
Download:
|
| Fig. 5.The 1H NMR spectra (400 MHz, D2O, 25 ℃) of Se-Cys (0.3 mmol/L) in the absence and presence of SC4A-H (1 mmol/L) at pD 7.0. ‘‘◆’’ represents DSS signals. | |
In order to investigate the redox responsiveness of the assembly,H2O2 was added into the SC4A-H/Se-Cys solutions because H2O2 can be used as an oxidant to cleave the Se-Se bonds in Se-Cys [34]. DLS experiments showed that,after the addition of H2O2 for 3 h,the intensity of the SC4A-H/Se-Cys assembly dropped from 133.3 kcps to 13.1 kcps,accompanied by a wide distribution of particle size. This result indicated that the Se-Se bonds in Se-Cys was cleaved by the oxidant H2O2,leading to the disassembly of the SC4A-H/Se-Cys nanoparticles. In addition to oxidants,reductants such as thiols and reduced glutathione (GSH) were also able to cleave the Se-Se bonds [32]. After the addition of 0.1 mg/mL GSH for 2 h,the DLS results of SC4A-H/Se-Cys solution showed no appreciable signals,indicating the complete disassembly of nanoparticles. These results jointly demonstrate the good responsiveness of SC4A-H/Se-Cys assembly toward oxidation or reduction stimuli.
It is also interesting to investigate the redox-reponsive release behaviors of SC4A-H/Se-Cys assembly to model substrates,because a very important characteristic feature of a delivery system is its controlled release ability to the loaded molecules. As described above,the loading of Nile Red into the interior of SC4AH/Se-Cys nanoparticles gave the strong fluorescence emission. With the addition of GSH as a reductant,the fluorescence intensity of Nile Red-load nanoparticles quenched 76% within 1 h (Fig. 6a and b),indicating the release of Nile Red from the hydrophobic nanoparticle interior to the aqueous solution due to the GSHresponsive disassembly of nanoparticles. Similar phenomenon was also observed in the case of Nile Red-load nanoparticles treated by H2O2 as an oxidant,where the fluorescence intensity of Nile Redload nanoparticles quenched 73% within 200 min (Fig. 6c and d). Therefore,we can draw the conclusion that the SC4A-H/Se-Cys nanoparticles can be used in the delivery and the controllable release of hydrophobic substrates via oxidation or reduction stimuli.

|
Download:
|
| Fig. 6. (a) Fluorescence spectra of Nile Red-loaded SC4A-H/Se-Cys nanoparticle solution treated by GSH (0.1 mg/mL) at 25 ℃ for 2 h, (b) dependence of the fluorescence intensity of Nile Red-loaded SC4A-H/Se-Cys nanoparticle solution at 650 nm on time. (c) Fluorescence spectra of Nile Red-loaded SC4A-H/Se-Cys nanoparticle solution treated by H2O2 (0.1%, v/v) at 25 ℃ for 5 h, and (d) dependence of the fluorescence intensity of Nile Red-loaded SC4A-H/Se-Cys nanoparticle solution at 650 nm on time. | |
In summary,a diselenide-containing nanoparticle was successfully constructed through the guest-induced aggregation of sulfonatocalixarene,showing good responsiveness to redox stimuli. After loading the hydrophobic substrates,the nanoparticles can be disrupted when treated by either oxidant or reductant to release the loaded substrates,which will provide an easy and effective way to fabricate diselenide-containing assembly and enable its potential application as a multi-responsive controlled delivery system.
Acknowledgments
We thank ‘‘973’’ Program (No. 2011CB 932502) and NNSFC (Nos. 91227107,21432004 and 21272125) for financial support.
| [1] | Y. Lu, W. Sun, Z. Gu, Stimuli-responsive nanomaterials for therapeutic protein delivery, J. Control. Release 194 (2014) 1–19. |
| [2] | S. Mura, J. Nicolas, P. Couvreur, Stimuli-responsive nanocarriers for drug delivery, Nat. Mater. 12 (2013) 991–1003. |
| [3] | E.G. Kelley, J.N.L. Albert, M.O. Sullivan, T.H. Epps, Stimuli-responsive copolymer solution and surface assemblies for biomedical applications, Chem. Soc. Rev. 42 (2013) 7057–7071. |
| [4] | J. Chen, X. Qiu, J. Ouyang, et al., pH and reduction dual-sensitive copolymeric micelles for intracellular doxorubicin delivery, Biomacromolecules 12 (2011) 3601–3611. |
| [5] | D.J. Fu, Y. Jin, M.Q. Xie, et al., Preparation and characterization of mPEG grafted chitosan micelles as 5-fluorouracil carriers for effective anti-tumor activity, Chin. Chem. Lett. 25 (2014) 1435–1440. |
| [6] | D. Han, X. Tong, Y. Zhao, Block copolymer micelles with a dual-stimuli-responsive core for fast or slow degradation, Langmuir 28 (2012) 2327–2331. |
| [7] | L.X. Yu, Y. Liu, S.C. Chen, Y. Guan, Y.Z. Wang, Reversible photoswitching aggregation and dissolution of spiropyranfunctionalized copolymer and light-responsive FRET process, Chin. Chem. Lett. 25 (2014) 389–396. |
| [8] | T. Ta, A.J. Convertine, C.R. Reyes, P.S. Stayton, T.M. Porter, Thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin, Biomacromolecules 11 (2011) 1915–1920. |
| [9] | G. von Maltzahn, T.J. Harris, J.H. Park, et al., Nanoparticle self-assembly gated by logical proteolytic triggers, J. Am. Chem. Soc. 129 (2007) 6064–6065. |
| [10] | L. Dong, S. Xia, Z. Huang, et al., A pH/enzyme-responsive tumor-specific delivery system for doxorubicin, Biomaterials 31 (2010) 6309–6316. |
| [11] | C. Wei, J. Guo, C. Wang, Dual stimuli-responsive polymeric micelles exhibiting “and” logic gate for controlled release of adriamycin, Macromol. Rapid Commun. 32 (2011) 451–455. |
| [12] | R. Boyd, Selenium stories, Nat. Chem. 3 (2011) 570. |
| [13] | G. Mugesh, W.W. du Mont, H. Sies, Chemistry of biologically important synthetic organoselenium compounds, Chem. Rev. 101 (2001) 2125–2180. |
| [14] | H. Xu, W. Cao, X. Zhang, Selenium-containing polymers: promising biomaterials for controlled release and enzymer minics, Acc. Chem. Res. 46 (2013) 1647–1658. |
| [15] | N.K. Kildahl, Bond energy data summarized, J. Chem. Educ. 72 (1995) 423–424. |
| [16] | N. Ma, Y. Li, H. Xu, Z. Wang, X. Zhang, Dual redox responsive assemblies formed from diselenide block copolymers, J. Am. Chem. Soc. 132 (2010) 442–443. |
| [17] | N. Ma, Y. Li, H. Ren, et al., Selenium-containing block copolymers and their oxidation-responsive aggregates, Polym. Chem. 1 (2010) 1609–1614. |
| [18] | W. Cao, Y. Li, Y. Yi, et al., Coordination-responsive selenium containing polymer micelles for controlled drug release, Chem. Sci. 3 (2012) 3403–3408. |
| [19] | N. Ma, H. Xu, L. An, et al., Radiation-sensitive diselenide block copolymer micellar aggregates: toward the combination of radiotherapy and chemotherapy, Langmuir 27 (2011) 5874–5878. |
| [20] | P. Han, N. Ma, H. Ren, et al., Oxidation-responsive micelles based on a seleniumcontaining polymeric superamphiphile, Langmuir 26 (2010) 14414–14418. |
| [21] | D.S. Guo, Y. Liu, Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications, Acc. Chem. Res. 47 (2014) 1925–1934. |
| [22] | D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, Cholinesterase-responsive supramolecular vesicle, J. Am. Chem. Soc. 134 (2012) 10244–10250. |
| [23] | K. Wang, D.S. Guo, X. Wang, Y. Liu, Multistimuli responsive supramolecular vesicles based on the recognition of p-sulfonato calixarene and its controllable release of doxorubicin, ACS Nano 5 (2011) 2880–2894. |
| [24] | B.P. Jiang, D.S. Guo, Y.C. Liu, K.P. Wang, Y. Liu, Photomodulated fluorescence of supramolecular assemblies of sulfonato calixarenes and tetraphenylethene, ACS Nano 8 (2014) 1609–1618. |
| [25] | C. Zhou, X. Chen, Y. Yun, J. Wang, J. Huang, Reversible transition between SDS@2β-CD microtubes and vesicles triggered by temperature, Langmuir 30 (2014) 3381–3386. |
| [26] | C. Zhou, X. Chen, Q. Zhao, et al., Self-assembly of nonionic surfactant tween 20@2β-CD inclusion complexes in dilute solution, Langmuir 23 (2013) 13175– 13182. |
| [27] | G. Yu, X. Zhou, Z. Zhang, et al., Pillar[6]arene/paraquat molecular recognition in water: high binding strength, pH-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning, J. Am. Chem. Soc. 134 (2012) 19489–19497. |
| [28] | X.Y. Hu, K. Jia, Y. Cao, et al., Dual pH- and photo-responsive supramolecular nanocarriers based on water-soluble pillar[6]arene and different azobenzene derivatives for intracellular anticancer drug delivery, Chem. Eur. J. (2015), http://dx.doi.org/10.1002/chem.201405095. |
| [29] | Y. Cao, X.Y. Hu, Y. Li, et al., Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release, J. Am. Chem. Soc. 136 (2014) 10762–10769. |
| [30] | J. Tian, T.Y. Zhou, S.C. Zhang, et al., Three-dimensional periodic supramolecular organic framework ion sponge in water and microcrystals, Nat. Commun. 5 (2014) 5574. |
| [31] | L.H. Wang, Z.J. Zhang, H.Y. Zhang, H.L. Wu, Y. Liu, A twin-axial[5]pseudorotaxane based on cucurbit[8]uril and α-cyclodextrin, Chin. Chem. Lett. 24 (2013) 949–952. |
| [32] | N. Basilio, L. Garcia-Rio, Calixarene-based surfactants: conformational-dependent solvation shells for the alkyl chains, ChemPhysChem 13 (2012) 2368–2376. |
| [33] | P.J.G. Coutinho, E.M.S. Castanheira, M.C. Rei, M.E.C.D. Real Oliveira, Nile red and DCM fluorescence anisotropy studies in C12E7/DPPC mixed systems, J. Phys. Chem. B 106 (2002) 12841–12846. |
| [34] | A. Fredga, Organic selenium chemistry, Ann. N. Y. Acad. Sci. 192 (1972) 1–9. |




