b College of Chemistry, Beijing Normal University, Beijing 100875, China;
c University of the Chinese Academy of Sciences, Beijing 100049, China
Pillararenes are a novel kind of macrocyclic host for supramolecular chemistry composed of hydroquinone units linked by methylene bridges at para positions [1, 2]. They have held the spotlight in this field owing to their unique highly rigid structure and easy functionalization with various substituents at the hydroquinone unit [3, 4, 5, 6]. Because of the electron-rich property, their cavities can encapsulate many types of cationic guests and neutral molecules [7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. These features make them good candidate to construct various assemblies with intriguing properties. Hitherto,many pillararene-based supramolecular architectures have been built,such as the first pillararene-based [c2]daisy chain containing two mirror image monomers reported by Huang’s group [17],[2]rotaxane whose motions could be triggered by solvent and temperature [18],and the double-switchable pillararene- based supramolecular polymer reported by our group [19].
Pseudorotaxanes,a self-assembly threaded structure in which macrocyclic components encircle linear components,have been investigated with great interest. They are the building blocks for the preparation of artificial molecular machines,supramolecular polymers,supramolecular gels,and other functional supramolecular materials [20, 21, 22, 23]. Some of them are promising in preparation of molecular sensors and in drug delivery [24, 25, 26, 27, 28]. [1]pseudorotaxanes are one of the basic threaded structures for pseudorotaxane. However,stable pillar[5]arene-based [1]pseudorotaxanes are still rarely reported because they mostly exist in dilute solutions mixt with linear oligomers,cyclic dimers and so on [29]. In 2011,Ogoshi et al. presented firstly the [1]pseudorotaxanes based on pillar[5]arene with octyltrimethylammonium group,which exhibited self-inclusion complexation in chloroform. But it was uncertain whether this [1]pseudorotaxane could form at high concentration due to the restriction of solubility [30]. Stoddart et al. investigated detailedly the assembling behavior of a monofunctionalized pillar[5]arene derivative containing a viologen moiety. It behaved as selfcomplexation at low concentration,assembled into supramolecular daisy chain polymers and eventually organogels upon the increasing of its concentration [31]. Herein,we report a stable pillar[5]arene-based [1]pseudorotaxane even in concentrated chloroform solution (100 mmol/L) by reasonable monofunctionalization. The self-complexation was demonstrated by the research of 1H NMR,2D COSY NMR,2D NOESY NMR,viscosity measurements and HR-ESI-MS. Furthermore,[1]rotaxane was obtained efficiently through thiol-ene reaction confirming the formation of [1]pseudorotaxane. 2. Experimental
All the experimental procedures including materials and methods,synthesis and characterization details,and copies of NMR spectra were present in Supporting information. 3. Results and discussion
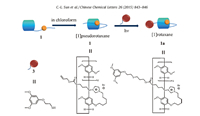
As shown in Scheme 1,monofunctionalized pillar[5]arene with imidazolium was exploited in our system due to the strong complexation (Ka = 1.0 × 104 L/mol) between pillar[5]arene and imidazolium cation [32]. To ensure the formation of selfcomplexation assemblies,there were two long alkyl chain linked to imidazolium. One of them was designed to provide the flexibility and the other one appeared as the guest part. Moreover, an allyl group was added at the end of the alkyl guest as a reactive site,which offered the possibility to prepare [1]rotaxane through thiol-ene reaction. Compound 1 was synthesized in five steps with moderate overall yield (Scheme S1 in Supporting information). By similar method,compound 2,which had the same linear guest part but without pillar[5]arene unit,was synthesized as a reference guest (Scheme S1).
|
Download:
|
| Scheme 1.Illustration of the self-complexation of monofunctionalized pillar[5]arene 1 and the formation of [1]rotaxane | |
The self-assembled behavior of 1 in solution was first examined by 1H NMR spectroscopy at 40 mmol/L in CDCl3. The 1H NMR spectrum of compound 1 showed quite different from compound 2 at the same concentration. The signals of protons on the imidazolium unit (H7,H8,H9) and alkyl chain (H10,H11,H12, H13) became broader and shifted upfield,including two obvious broadened peaks (H11,H12) located at minus ppm. These upfield shifts resulted from the threading of imidazolium guest into the pillar[5]arene macrocycle (Fig. 1). In order to confirm the proton assignments clearly,two-dimensional correlation spectroscopy 1H NMR (COSY) was performed in combination with 1H NMR (Fig. S5 in Supporting information). It was demonstrated that the signals of protons H7-H12 on or next to the imidazolium ring revealed more than 2 ppm upfield chemical shift,and those of H13,H14 showed a fraction of change,while the protons on the other alkyl chain showed little change. This result indicated that imidazolium moiety and adjacent alkyl chain,the one distant from pillar[5]- arene unit,were threaded into the electron-rich cavity and the other protons were located outside the cavity. In addition,the NOESY analyst was consistent with this result in which strong NOE correlation signals were presented between the protons H10,H11, H12,H13 and the aromatic or methylene hydrogen atoms of pillar[5]arene (Fig. S6 in Supporting information).

|
Download:
|
| Fig. 1.1H NMR (40 mmol/L in CDCl3, 400 MHz) spectrum and structure of 1 (a), and 2 (b). | |
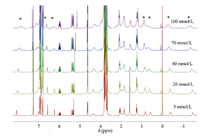
The above spectra validated the complexation of compound 1 undoubtedly,but could not supply us the exact information about whether the assembly was intra- or inter-molecule. To verify the structure of [1]pseudorotaxane,the 1H NMR spectroscopy of compound 1 at variable concentration (from 5 mmol/L to 100 mmol/L) were measured,which did not show any apparent changes (Fig. 2). Along with the increase of concentration,only some signals gave slight broadened trend,indicating that compound 1 did not form intermolecular complexes in CDCl3.
|
Download:
|
| Fig. 2.Partial 1H NMR (CDCl3, 400 MHz) spectra of 1 at different concentrations (the peaks of complexed part are denoted by asterisks). | |
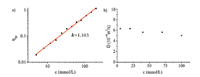
The self-complexation behavior of 1 in chloroform was further studied by viscosity measurements. As presented in Fig. 3a,the curve in the double logarithmic plots of specific viscosity vs. concentration at 5-200 mmol/L had a slope of 1.103,suggesting a linear relationship between specific viscosity and concentration, which is characteristic for non-interacting assemblies of constant size,in agreement with the intramolecular-threading even at high concentrations [33]. Meanwhile,two-dimensional diffusion-ordered 1H NMR (DOSY) experiments were also employed to investigate the self-assembly of compound 1 at different concentrations (Fig. 3b). The DOSY spectrum at every concentration revealed one set of signals,manifesting only one aggregate size in solutions. As the concentration of 1 increased from 5 mmol/L to 100 mmol/L,the value of weight average diffusion coefficients (D) decreased from 6.28 × 10-10 to 5.02 × 10-10m2/s (D5/D100 = 1.25). This slight decrease was insufficient to suggest the change of the average dimension of aggregates. That was to say as the concentration increased,the self-assembly kept the same. The high resolution ESI mass spectrometry of a mixture of 1 revealed the molecular peak at m/z 1041.5331 corresponding to the monomer,backing up the result of intramolecular complexation (Fig. S1c in Supporting information).
|
Download:
|
| Fig. 3.(a) Specific viscosity of 1 in CHCl3 solutions vs. the concentration (293 K). (b) Concentration dependence of diffusion coefficient D (CDCl3, 298 K, 600 MHz) of 1. | |
The above result indicated the existed complexation but no intermolecular aggregates in the chloroform solution of compound 1 at the concentration of 5-100 mmol/L. It was reasonably deduced that stable [1]pseudorotaxane was formed by the new monofunctionalized pillar[5]arene,which was different from the usual assembly as reported by literature when bearing such a long side chain [17, 34, 35, 36]. It was probably because this molecule held two long flexible chains linked to imidazolium,which ensured the intramolecular interaction between the alkyl chain distant from the pillar[5]arene and the pillar[5]arene cavity.
Finally,the formation of [1]pseudorotaxane was proved considerably by the preparation of [1]rotaxane. Considering the terminal group of compound 1,we came up with the photo thiolene radical reaction. As an important member of thiol-click family, thiol-ene reaction possessed the merits of facile condition,rapid reaction rate,excellent yield,along with strong tolerance for many kinds of functional groups [37]. Here,compound 3 was selected as the stopper and dichloromethane was employed as the solvent (Scheme 1). [1]Rotaxane was obtained successfully both at the concentration of 20 mmol/L and 100 mmol/L in relatively high isolated yield (57% and 54%) (Scheme S2 in Supporting information). Fig. S7 in Supporting information showed the 1HNMR spectra of [1]pseudorotaxane and [1]rotaxane. Except the new peaks from the added stopper as well as the disappearance of double bond signals,1H NMR spectrum kept almost same after reaction, demonstrating no change of complexation. Besides,we prepared the control compound 2a through thiol-ene reaction between 2 and 3 (Scheme S2). In comparison with the 1H NMR spectrum of 2a,the assembly of 1a was clear (Fig. S8 in Supporting information). In this regard,the convenience and efficiency of thiol-ene reaction might supply a new synthesis strategy for rotaxane. 4. Conclusion
In summary,we described a stable monofunctionalized pillar[5]arene-based [1]pseudorotaxane whose self-inclusion structure was exhaustively proved via varying concentration 1H NMR,2D COSY NMR,2D NOESY NMR,viscosity measurements,2D DOSY NMR,and HR-ESI-MS analysis. [1]Rotaxane,which was prepared efficiently through thiol-ene reaction confirmed the formation of the stable [1]pseudorotaxane. We suspect that this [1]pseudorotaxane and the corresponding [1]rotaxane are potential in developing novel functional molecular machines.
Acknowledgments
We are grateful for the financial support from the 973 Program (No. 2013CB933800),the National Natural Science Foundation of China (Nos. 21222210,21474124,21472202).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2015. 05.030.
| [1] | T. Ogoshi, S. Kanai, S. Fujinami, T.A. Yamagishi, Y. Nakamoto, para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host–guest property, J. Am. Chem. Soc. 130 (2008) 5022–5023. |
| [2] | D.R. Cao, Y.H. Kou, J.Q. Liang, et al., A facile and efficient preparation of pillararenes and a pillarquinone, Angew. Chem. Int. Ed. 48 (2009) 9721–9723. |
| [3] | M. Xue, Y. Yang, X.D. Chi, Z.B. Zhang, F.H. Huang, Pillararenes, a new class of macrocycles for supramolecular chemistry, Acc. Chem. Res. 45 (2012) 1294–1308. |
| [4] | H.C. Zhang, Y.L. Zhao, Pillararene-based assemblies: design principle, preparation and applications, Chem. Eur. J. 19 (2013) 16862–16879. |
| [5] | T. Ogoshi, T. Yamagishi, Pillar[5]- and pillar[6]arene-based supramolecular assemblies built by using their cavity-size-dependent host–guest interactions, Chem. Commun. 50 (2014) 4776–4787. |
| [6] | H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545–552. |
| [7] | T. Ogoshi, S. Tanaka, T. Yamagishi, Y. Nakamoto, Ionic liquid molecules (ils) as novel guests for pillar[5]arene: 1:2 host–guest complexes between pillar[5]arene and ILs in organic media, Chem. Lett. 40 (2011) 96–98. |
| [8] | N.L. Strutt, R.S. Forgan, J.M. Spruell, Y.Y. Botros, J.F. Stoddart, Monofunctionalized pillar[5]arene as a host for alkanediamines, J. Am. Chem. Soc. 133 (2011) 5668– 5671. |
| [9] | X.Y. Wang, K. Han, J. Li, X.S. Jia, C.J. Li, Pillar[5]arene-neutral guest recognition based supramolecular alternating copolymer containing [c2] daisy chain and bispillar[5]arene units, Polym. Chem. 4 (2013) 3998–4003. |
| [10] | L.L. Tan, Y.M. Zhang, B. Li, et al., Selective recognition of "solvent" molecules in solution and the solid state by 1:4-dimethoxypillar[5]arene driven by attractive forces, New J. Chem. 38 (2014) 845–851. |
| [11] | Y.J. Zhou, Z.T. Li, X.D. Chi, C. Thompson, Y. Yao, Formation of a [2]pseudorotaxane based on a pillar[5]arene and a rigid guest in solution and in the solid state, Chem. Commun. 50 (2014) 10482–10484. |
| [12] | C.J. Li, K. Han, J. Li, et al., Supramolecular polymers based on efficient pillar[5]- arene neutral guest motifs, Chem. Eur. J. 19 (2013) 11892–11897. |
| [13] | X.S. Hu, H.M. Deng, J. Li, X.S. Jia, C.J. Li, Selective binding of unsaturated aliphatic hydrocarbons by a pillar[5]arene, Chin. Chem. Lett. 24 (2013) 707–709. |
| [14] | M. Pan, M. Xue, Cover picture: synthesis of a pillar[5]arene with both hydroxyl and methoxycarbonyl-methoxy groups and its host–guest complexation with a bis(imidazolium) salt, Chin. J. Chem. 32 (2014) 109. |
| [15] | X. Lou, H. Chen, X. Jia, C. Li, Complexation of linear aliphatic ester, aldehyde and ketone guests by per-ethylated pillar[5]arene, Chin. J. Chem. 33 (2015) 335–338. |
| [16] | H. Huang, L. Liu, W. Duan, Y. Huang, G. Lin, Synthesis of copillar[5]arenes and their host–guest complexation with two types of guests, Chin. J. Chem. 33 (2015) 384– 388. |
| [17] | Z.B. Zhang, G.C. Yu, C.Y. Han, et al., Formation of a cyclic dimer containing two mirror image monomers in the solid state controlled by van der Waals forces, Org. Lett. 13 (2011) 4818–4821. |
| [18] | S.Y. Dong, J.Y. Yuan, F.H. Huang, A pillar[5]arene/imidazolium [2]rotaxane: solvent- and thermo-driven molecular motions and supramolecular gel formation, Chem. Sci. 5 (2014) 247–252. |
| [19] | J.F. Xu, Y.Z. Chen, L.Z. Wu, C.H. Tung, Q.Z. Yang, Dynamic covalent bond based on reversible photo [4 + 4] cycloaddition of anthracene for construction of doubledynamic polymers, Org. Lett. 15 (2013) 6148–6151. |
| [20] | C.Y. Cheng, P.R. McGonigal, W.G. Liu, et al., Energetically demanding transport in a supramolecular assembly, J. Am. Chem. Soc. 136 (2014) 14702–14705. |
| [21] | N.K. Jena, N.A. Murugan, Solvent-dependent conformational states of a [2]rotaxane- based molecular machine: a molecular dynamics perspective, J. Phys. Chem. C 117 (2013) 25059–25068. |
| [22] | C. Gaeta, C. Talotta, P. Neri, Pseudorotaxane orientational stereoisomerism driven by pi-electron density, Chem. Commun. 50 (2014) 9917–9920. |
| [23] | H. Chen, X. Jia, C. Li, A pillar[6]arene-[2]pseudorotaxane based pH-sensitive molecular switch, Chin. J. Chem. 33 (2015) 343–345. |
| [24] | M.J. Langton, O.A. Blackburn, T. Lang, S. Faulkner, P.D. Beer, Nitrite-templated synthesis of lanthanide-containing [2]rotaxanes for anion sensing, Angew. Chem. Int. Ed. 53 (2014) 11463–11466. |
| [25] | S. Sun, J.B. Shi, Y.P. Dong, et al., A pillar[5]arene-based side-chain pseudorotaxanes and polypseudorotaxanes as novel fluorescent sensors for the selective detection of halogen ions, Chin. Chem. Lett. 24 (2013) 987–992. |
| [26] | S. Sun, X.Y. Hu, D.Z. Chen, et al., Pillar[5]arene-based side-chain polypseudorotaxanes as an anion-responsive fluorescent sensor, Polym. Chem. 4 (2013) 2224–2229. |
| [27] | M.H. Li, Y. Yan, C. Teh, V. Korzh, Y.L. Zhao, NIR-triggered drug release from switchable rotaxane-functionalized silica-covered Au nanorods, Chem. Commun. 50 (2014) 9745–9748. |
| [28] | L.H. Wang, Z.J. Zhang, H.Y. Zhang, H.L. Wu, Y. Liu, A twin-axial[5]pseudorotaxane based on cucurbit[8]uril and alpha-cyclodextrin, Chin. Chem. Lett. 24 (2013) 949– 952. |
| [29] | M.F. Ni, X.Y. Hu, J.L. Jiang, L.Y. Wang, The self-complexation of mono-ureafunctionalized pillar[5]arenes with abnormal urea behaviors, Chem. Commun. 50 (2014) 1317–1319. |
| [30] | T. Ogoshi, K. Demachi, K. Kitajima, T. Yamagishi, Monofunctionalized pillar[5]- arenes: synthesis and supramolecular structure, Chem. Commun. 47 (2011) 7164–7166. |
| [31] | N.L. Strutt, H.C. Zhang, M.A. Giesener, J.Y. Lei, J.F. Stoddart, A self-complexing and self-assembling pillar[5]arene, Chem. Commun. 48 (2012) 1647–1649. |
| [32] | B.Y. Xia, B. Zheng, C.Y. Han, et al., A novel pH-responsive supramolecular polymer constructed by pillar[5]arene-based host–guest interactions, Polym. Chem. 4 (2013) 2019–2024. |
| [33] | Y.L. Liu, Z.Q. Wang, X. Zhang, Characterization of supramolecular polymers, Chem. Soc. Rev. 41 (2012) 5922–5932. |
| [34] | Z.B. Zhang, Y. Luo, J.Z. Chen, et al., Formation of linear supramolecular polymers that is driven by C–H center dot center dot center dot pi interactions in solution and in the solid state, Angew. Chem. Int. Ed. 50 (2011) 1397–1401. |
| [35] | Z. Zhang, C. Han, G. Yu, F. Huang, A solvent-driven molecular spring, Chem. Sci. 3 (2012) 3026. |
| [36] | L.Z. Liu, L.Y. Wang, C.C. Liu, et al., Dimerization control in the self-assembly behavior of copillar[5]arenes bearing omega-hydroxyalkoxy groups, J. Org. Chem. 77 (2012) 9413–9417. |
| [37] | C.E. Hoyle, A.B. Lowe, C.N. Bowman, Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis, Chem. Soc. Rev. 39 (2010) 1355–1387. |




