Molecular clips [1] are generally composed of a tether and two flat,aromatic pincers. During the past two decades,they have drawn much attention for their specific structures,convenient synthesis and potential applications in supramolecular chemistry. However,examples for chiral molecular clips [2] are still rare.
Tröger’s bases [3] are a kind of rigid,chiral V-shaped molecules containing a diazocine ring conjugated to two aromatic moieties. Their specific structural properties made them to be found more and more applications in supramolecular chemistry,molecular catalysis,and column separation process. By using Tröger’s base as a chiral linking unit,Wilcox et al. reported the first chiral molecular clip with a deep cleft large enough to bind small organic molecules in 1987 [4]. However,since the aromatic pincer of the molecule clip was the adduct of anthracene and dimethyl acetylenedicarboxylate, three diastereomeric Tröger’s bases with a 1:2:1 ratio could be yielded. Moreover,it was also difficult for the molecular clips to be further derivated.
Triptycene [5] with a unique three-dimensional rigid structure has been proved to be a useful building block for the design and synthesis of novel macrocyclic hosts [6]. Based on the triptycene building block,molecular tweezers or clips could also be synthesized by different structural motif [7]. Since aminosubstituted triptycene derivatives can be easily available [8, 9], we deduced that triptycene with concave aromatic surface could be linked by Tröger’s base unit for developing new chiral rigid molecular clips with potential applications in enantioselective recognition and asymmetric catalysis. Herein,we report the convenient and efficient synthesis of a series of rigid molecular clips based on triptycene-derived Tröger’s bases,which were characterized by NMR,MS spectra,and X-ray crystal structure. 2. Experimental 2.1. Typical preparation procedure and characterizations for nitrosubstituted triptycenes 2a-2c
To a gently refluxing solution of the anthracene (1a-1c) (1 mmol) in 1,2-dichloroethane (50 mL) was added portionwise 5-nitrobenzenediazonium-2-carboxylate (2.5 mmol). When TLC showed that the starting anthracene was consumed,the reaction mixture was concentrated under reduce pressure. The residue was purified by flash column chromatography on silica gel with petroleum ether: CH2Cl2 = 4:1 (v/v) as eluent to yield the triptycene as a pale yellow solid.
2a [9]: 108 mg,36% yield. 1H NMR (300 MHz,CDCl3): δ 8.21 (d, 1H,J = 2.1 Hz),7.93 (dd,1H,J = 8.1,2.1 Hz),7.49 (d,1H,J = 8.1 Hz), 7.44-7.40 (m,4H),7.06-7.03 (m,4H),5.54 (s,2H).
2b: 67 mg,15% yield. 1H NMR (300 MHz,CDCl3): δ 8.08 (s,1H), 7.87 (d,1H,J = 6.9 Hz),7.37 (d,1H,J = 8.2 Hz),6.98 (s,2H),6.97 (s,2H),3.86 (s,12H),2.46 (s,3H),2.43 (s,3H). 13C NMR (75 MHz,CDCl3): δ 156.4,151.2,146.3,146.2,145.1,140.4,139.9,120.9, 120.4,115.0,106.2,106.0,56.42,56.38,48.5,48.3,13.9,13.7. EITOF- MS: m/z 447 [M]+. Anal. calcd. for C26H25NO6•0.1CH2Cl2: C, 68.75; H,5.57; N,3.07. Found: C,68.81; H,5.80; N,2.97.
2c: 91 mg,18% yield. 1H NMR (300 MHz,CDCl3): δ 8.14 (s,1H), 7.90 (d,1H,J = 6.8 Hz),7.41 (d,1H,J = 8.2 Hz),7.00 (s,2H),6.99 (s, 2H),3.85 (s,12H),2.93-2.85 (m,4H),2.26-2.16 (m,4H),1.50-1.42 (m,6H). 13C NMR (75 MHz,CDCl3): δ 145.9,145.8,144.7,140.2, 139.8,121.6,120.5,116.4,107.5,56.32,56.26,53.2,52.9,30.5,30.4, 18.94,18.92,16.1. EI-TOF-MS: m/z 503 [M]+. Anal. calcd. for C30H33NO6•0.05CH2Cl2: C,71.07; H,6.57; N,2.76. Found: C,71.00; H,6.55; N,2.92. 2.2. Typical preparation procedure and characterizations for aminosubstituted triptycenes 3a-3c
To the mixture of nitro-substituted triptycene 2a-2c (2.5 mmol) and 10% Pd/C (27 mg,0.25 mmol) in MeOH (25 mL) and dichloromethane (25 mL) under Ar atmosphere was flushed with H2. When TLC showed that the starting material was consumed,the reaction mixture was filtered through a plug of celite,and the celite was washed with dichloromethane. The combined filtrate was concentrated under reduce pressure to give the product.
3a [9]: 266 mg,99% yield. 1H NMR (300 MHz,CDCl3): δ 7.35- 7.32 (m,4H),7.14 (d,1H,J = 7.8 Hz),6.98-6.95 (m,4H),6.81 (d,1H, J = 2.2 Hz),6.30 (dd,1H,J = 7.7,2.2 Hz),5.30 (s,1H),5.28 (s,1H), 3.85 (s,2H).
3b: 400 mg,96% yield. 1H NMR (300 MHz,CDCl3): δ 7.05 (d,1H, J = 7.9 Hz),6.91 (s,4H),6.69 (d,1H,J = 2.0 Hz),6.26 (dd,1H,J = 7.9, 2.0 Hz),3.82 (s,12H),3.36 (s,2H),2.32 (s,3H),2.31 (s,3H). 13CNMR (75 MHz,CDCl3): δ 150.3,145.8,145.6,143.2,142.2,141.5,139.7, 120.6,110.2,108.8,106.1,105.8,56.5,56.4,47.9,47.3,13.9. EITOF- MS: m/z 417 [M]+. HRMS-ESI calcd. for C26H27NO4: 417.1940. Found: 417.1947.
3c: 464 mg,98% yield. 1H NMR (300 MHz,CDCl3): δ 7.07 (d,1H, J = 8.1 Hz),6.94 (s,4H),6.71 (s,1H),6.24 (d,1H,J = 7.8),3.82 (s, 12H),3.36 (s,2H),2.80-2.75 (m,4H),2.20-2.16 (m,4H),1.42-1.36 (m,6H). 13C NMR (75 MHz,CDCl3): δ 145.5,145.2,142.9,142.2, 122.1,110.2,110.0,107.7,107.3,56.40,56.35,52.6,52.0,30.83, 30.78,19.0,16.19,16.18. EI-TOF-MS: m/z 473 [M]+. HRMS-ESI calcd. for C30H35NO4: 473.2566. Found: 473.2572. 2.3. Typical preparation procedure and characterizations for triptycene-derived Tröger’s bases 4a-4c
To a solution of the amino-substituted triptycene 3a-3c (1 mmol) in TFA (10 mL) at 0℃ under Ar atmosphere was added paraformaldehyde (4 mmol). The reaction mixture was stirred at room temperature for 2 days,then poured into a mixture of ice and concentrated ammonia solution. The alkaline solution was extracted with dichloromethane (3×50 mL),and the combined organic extracts were successively washed with saturated aqueous NaHCO3 (100 mL) and NaCl (100 mL). The organic layer was dried over anhydrous MgSO4,evaporated under reduced pressure,and purified by flash column chromatography on silica gel to give the product.
4a: 402 mg,70% yield. 1H NMR (300 MHz,CDCl3): δ 7.34-7.28 (m,6H),7.22-7.20 (m,2H),7.07 (s,2H),6.96-6.93 (m,4H),6.90- 6.86 (m,4H),6.82 (s,2H),5.29 (s,2H),5.21 (s,2H),4.49 (d,2H, J = 16.5 Hz),4.11 (s,2H),3.97 (d,2H,J = 16.6 Hz). 13C NMR (75 MHz,CDCl3): δ 145.1,145.0,144.9,144.7,144.1,140.6, 125.3,125.24,125.20,125.1,124.0,123.6,123.5,123.42,123.39, 121.8,120.4,66.8,58.2,53.7,53.3. MALDI-TOF-MS: m/z 575.2 [M+H]+. Anal. calcd. for C43H30N2•0.7CH2Cl2: C,82.77; H, 4.99; N,4.42. Found: C,82.96; H,5.00; N,4.42.
4b: 792 mg,91% yield. 1H NMR (300 MHz,CDCl3): δ 6.99 (s,2H), 6.89 (s,2H),6.86 (s,4H),6.77 (s,2H),6.73 (s,2H),4.51 (d,2H, J = 16.5 Hz),4.12 (s,2H),3.98 (d,2H,J = 16.6 Hz),3.81-3.73 (m, 24H),2.31 (s,6H),2.23 (s,6H). 13C NMR (75 MHz,CDCl3): δ 148.2, 145.95,145.92,145.87,145.7,144.7,144.1,141.4,141.3,141.0, 123.2,118.3,117.1,106.11,106.06,105.8,105.5,66.9,58.3,56.49, 56.46,56.40,56.1,47.6,47.4,13.8. MALDI-TOF MS: m/z 871.4 [M+H]+. Anal. calcd. for C55H54N2O8•0.2CH2Cl2: C,74.66; H,6.17; N,3.15. Found: C,74.56; H,6.07; N,3.18.
4c: 796 mg,81% yield. 1H NMR (300 MHz,CDCl3): δ 7.00 (s,2H), 6.93 (s,2H),6.90 (s,4H),6.83 (s,2H),6.73 (s,2H),4.51 (d,2H, J = 16.5 Hz),4.10 (s,2H),3.94 (d,2H,J = 16.6 Hz),3.82-3.74(m,24H), 2.80-2.67 (m,8H),2.18-2.09 (m,8H),1.44-1.32 (m,12H). 13CNMR (75 MHz,CD3COCD3-d6): δ 149.3,146.8,146.7,146.5,145.1,142.4, 123.8,120.2,119.3,109.4,109.2,67.7,59.0,56.82,56.81,56.7,56.6, 53.1,52.9,31.3,19.6,19.5,16.3,16.2. MALDI-TOF-MS: m/z 983.8 [M+H]+. Anal. calcd. for C63H70N2O8•0.03CH2Cl2: C,76.79; H,7.16; N,2.84. Found: C,76.70; H,7.09; N,2.90. 2.4. Crystal data for 4b
CCDC 889458; C55H54N2O8,colorless,M = 871.00,orthorhombic, space group Pbca. a = 29.104 (11),b = 10.858 (4),c = 29.860 (11)Å ; V = 9436 (6)Å3; α = 90.00°,β = 90.00°,γ = 90.00°. Z = 8, T = 173 K,F000 = 3696,R1 = 0.0956,wR2 = 0.2481. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving. html or from the Cambridge Crystallographic Data Center,12 Union Road,Cambridge CB21EZ,UK; fax: +44 1223 336033; or deposit@ ccdc.cam.ac.uk. 3. Results and discussion
Synthetic routes of triptycene-derived Tröger’s bases 4a and 4b-c were outlined in Schemes 1 and 2,respectively. According to the published procedure [10],compound 2a was prepared in 36% yield by the Diels-Alder addition of 5-nitrobenzenediazonium-2- carboxylate to anthracene 1a in anhydrous 1,2-dichloroethane under argon. Similarly,starting from methoxyl-substituted triptycenes 1b-c,compounds 2b-c were prepared in 15% and 18%, respectively. Compound 2a could also be directly prepared by the nitration of triptycene by concentration nitric acid [9a]. However, under the similar reaction conditions,triptycene O-quinone derivatives instead of N-substituted triptycenes 2b-c could be obtained from methoxyl-substituted triptycenes [11]. With 2a in hand,compound 3a was synthesized in 99% yield by hydrogen reduction of 2a with 10% Pd/C in MeOH/THF. Finally,reaction of 3a with 4 equiv. of paraformaldehyde in trifluoroacetic acid gave compound 4a in 70%. According to the similar method,Tröger’s bases 4b-c were synthesized in 91% and 81% yield,respectively (Scheme 2). It was found that Tröger’s bases 4a-c have good solubility in common solvents including chlorinated solvents,THF, DMF,and even aqueous acetonitrile (CH3CN:H2O = 1:1,v/v).

|
Download:
|
| Scheme 1.Synthesis of triptycene-derived Tröger’s base 4a. | |

|
Download:
|
| Scheme 2.Synthesis of triptycene-derived Tröger’s bases 4b–c. | |
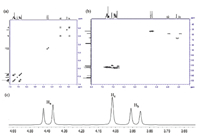
The structure of 4a was first confirmed by its 1H NMR and 13C NMR spectra. By the 1H-1H COSY spectrum (Fig. 1a) and HSQC spectrum (Fig. 1b) of 4a,the signals for the bridgehead protons of the triptycene subunits,the bridging methylene protons (Ha-Hc), and the corresponding carbons could be unambiguously assigned. Consequently,it was found that proton Hc showed one singlet at 4.07 ppm,while the bridgehead protons of the triptycene subunits showed two singlets at 5.20 and 5.28 ppm,respectively. Especially, the bridging methylene protons Ha and Hb showed two doublets at δ 3.93 and δ 4.44,respectively (Fig. 1c),indicating that protons Ha and Hb were magnetic nonequivalent due to the rigid structure of 4a. Moreover,one signal for the two bridging methylene carbons (ArCH2N),while two signals for the four bridgehead protons of the triptycene subunits were found. These observations indicated that molecule 4a has a C2 symmetric chiral structure. For compounds 4b and 4c,similar structural properties were also exhibited the detail please see the Supporting information.
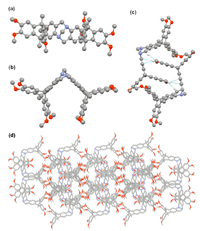
Structure of the Tröger’s base was further determined by the single crystal X-ray analysis. Consequently,the single crystal of 4b suitable for X-ray diffraction was obtained by slow evaporation of n-hexane to the CH2Cl2 solution of 4b. As shown in Fig. 2a and b, compound 4b shows a molecular clip shaped structure,and the Tröger’s base unit and the triptycene units in the molecular clip defined a concave surface of chiral structure,which is consistent with the NMR analysis. The clip shaped molecule showed a slight twist structure,and the dihedral angle between the two benzene rings linked by the nitrogen atoms of the Tröger’s base unit is 89.46°. The distance between two bridge carbons of the two triptycene moieties was found to be 9.143Å . Moreover,it was also found that two molecules of 4b could form a self-complementary dimer by a couple of C-H•••O interactions (dC-H•••O = 2.582Å ),and two pairs of C-H•••π interactions (dC-H•••π = 2.689 and 2.713Å ),in which a o-dimethoxybenzene unit of one molecular clip was situated at the cavity of another molecular clip,and vice versa (Fig. 2c). Furthermore,molecule 4b could self-assemble into a 3D microporous network by the packing of the dimer (Fig. 2d).
|
Download:
|
| Fig. 1.(a) 1H–1H COSY spectrum, (b) HSQC spectrum, and (c) partial 1H NMR spectrum (300 MHz, CDCl3) of 4a. | |
|
Download:
|
| Fig. 2.(a) Top view and (b) side view of the crystal structure of 4b. (c) Selfcomplementary dimer and (d) crystal packing of 4b. Solvent molecules and hydrogen atoms are omitted for clarity. | |
In conclusion,we have efficiently synthesized a series of triptycene-derived Tröger’s bases,and their structures were determined by 1H NMR,13C NMR,and MALDI-TOF MS spectra. The X-ray crystal structure further showed that the triptycenederived Tröger’s bases took chiral molecular clip-like structures. These chiral molecular clips might be found potential applications in enantioselective recognition and asymmetric catalysis,which are underway in our laboratory.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21332008,51373180),and the National Basic Research Program (No. 2011CB932501) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01.031.
| [1] | (a) F.G. Klärner, B. Kahlert, Molecular tweezers and clips as synthetic receptors. Molecular recognition and dynamics in receptor–substrate complexes, Acc. Chem. Res. 36 (2003) 919–932; (b) M. Hardouin–Lerouge, P. Hudhomme, M. Sallé, Molecular clips and tweezers hosting neutral guests, Chem. Soc. Rev. 40 (2011) 30–43. |
| [2] | B. Dolenský, J. Kessler, M. Jakubek, et al., Synthesis and characterisation of a new naphthalene tris-Tröger's base derivative –a chiral molecular clip, Tetrahedron Lett. 54 (2013) 308–311. |
| [3] | (a) B. Dolenský, M. Havlík, V. Král, Oligo Tröger's bases-new molecular scaffolds, Chem. Soc. Rev. 41 (2012) 3839–3858; (b) S. Sergeyev, Recent developments in synthetic chemistry, chiral separations, and applications of Tröger's base analogues, Helv. Chim. Acta 92 (2009) 415–444; (c) M. ValÍk, R.M. Strongin, V. Král, Tröger's base derivatives-new life for old compounds, Supramol. Chem. 17 (2005) 347–367. |
| [4] | C.S. Wilcox, L.M. Greer, V. Lynch, Synthesis of chiral molecular clefts. New armatures for biomimetic systems, J. Am. Chem. Soc. 109 (1987) 1865–1867. |
| [5] | (a) C.F. Chen, Y.X. Ma, Iptycene Chemistry: From Synthesis to Applications, Springer-Verlag, Berlin, Heidelberg, 2013; (b) C. Zhang, Y. Liu, X.Q. Xiong, et al., Three-dimensional nanographene based on triptycene: synthesis and its application in fluorescence imaging, Org. Lett. 14 (2012) 5912–5915; (c) C. Zhang, L.H. Peng, B. Li, et al., Organic microporous polymer from a hexaphenylbenzene based triptycene monomer: synthesis and its gas storage properties, Polym. Chem. 4 (2013) 3663–3666; (d) C. Zhang, Y. Liu, B. Li, et al., Triptycene-based microporous polymers: synthesis and their gas storage properties, ACS Macro Lett. 1 (2012) 190–193. |
| [6] | (a) C.F. Chen, Novel triptycene-derived hosts: synthesis and their applications in supramolecular chemistry, Chem. Commun. 47 (2011) 1674–1688; (b) Y. Han, Z. Meng, Y.X. Ma, C.F. Chen, Iptycene-derived crown ether hosts for molecular recognition and self-assembly, Acc. Chem. Res. 47 (2014) 2026–2040. |
| [7] | (a) X.X. Peng, H.Y. Lu, T. Han, C.F. Chen, Synthesis of a novel triptycene-based molecular tweezer and its complexation with paraquat derivatives, Org. Lett. 9 (2007) 895–898; (b) J. Cao, X.Z. Zhu, C.F. Chen, Synthesis, structure, and binding property of pentiptycene-based rigid tweezer-like molecules, J. Org. Chem. 75 (2010) 7420–7423; (c) Y. Jiang, J. Cao, J.M. Zhao, J.F. Xiang, C.F. Chen, Synthesis of a triptycene-derived bisparaphenylene-34-crown-10 and its complexation with both paraquat and cyclobis(paraquat-p-phenylene), J. Org. Chem. 75 (2010) 1767–1770; (d) T. Han, C.F. Chen, A triptycene-based bis(crown ether) host: complexation with both paraquat derivatives and dibenzylammonium salts, Org. Lett. 8 (2006) 1069–1072. |
| [8] | C. Zhang, C.F. Chen, Synthesis and structure of 2,6,14- and 2,7,14-trisubstituted triptycene derivatives, J. Org. Chem. 71 (2006) 6626–6629. |
| [9] | (a) J.H. Chong, M.J. MacLachlan, Robust non-interpenetrating coordination frameworks from new shape-persistent building blocks, Inorg. Chem. 45 (2006) 1442–1444; (b) J.H. Chong, M.J. MacLachlan, Synthesis and structural investigation of new triptycene-based ligands: en route to shape-persistent dendrimers and macrocycles with large free volume, J. Org. Chem. 72 (2007) 8683–8690. |
| [10] | (a) X.Z. Zhu, C.F. Chen, A highly efficient approach to [4]pseudocatenanes by threefold metathesis reactions of a triptycene-based tris[2]pseudorotaxane, J. Am. Chem. Soc. 127 (2005) 13158–13159; (b) Y. Han, Y. Jiang, C.F. Chen, Solid state self-assembly of triptycene-based catechol derivatives by multiple O–H…O hydrogen bonds, Chin. Chem. Lett. 24 (2013) 475–478. |
| [11] | J.M. Zhao, H.Y. Lu, J. Cao, Y. Jiang, C.F. Chen, Highly selective synthesis of triptycene O-quinone derivatives and their optical and electrochemical properties, Tetrahedron Lett. 50 (2009) 219–222. |




