In recent years,the construction of structurally well-defined architectures with good functions employing supramolecular amphiphiles has received increasing attention in chemistry,biology, materials and many other fields [1]. As compared with the traditional amphiphiles,supramolecular amphiphiles possess the advantages of the easy preparation,adjustive aggregation behaviour and multistimuli responsive properties towards light,temperature, redox,pH and enzyme [2]. Recently,various macrocyclic receptors, including calixarene [3],pillararene [4],and cucurbituril [5] were widely reported to induce the supramolecular amphiphilic aggregation of guest molecules. However,the related works based on cyclodextrins (CDs) are still rare,despite that CDs are regarded as one of the best building blocks for the construction of functional molecular assemblies [6]. Zhang et al. [2a] reported a photocontrolled reversible supramolecular assembly of an azobenzenecontainingsurfactantwith α- CD. Lauth-deViguerie et al. [7] reported the formation and physicochemical properties of noncovalent amphiphiles between β-CD and aliphatic guests. In the present work,we synthesized a two positively charged β-CD derivatives bearing one or seven methylimidazoliumyl arms (Scheme 1) and compared their molecular induced aggregation behaviours towards two anionic surfactants possessing the same hydrophilic head and the different hydrophobic tails (Scheme 2) by UV-vis,NMR,Zetapotential, dynamic light scattering (DLS),and transmission electron microscopy. It is our particular interest to explore the governing factors on the molecular assembly of amphiphilic substrates induced by modified CDs and to open a new approach for the CDbased supramolecular assembly.
|
Download:
|
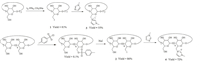
| Scheme 1.Synthesis route of hosts 3 and 4. | |
|
Download:
|
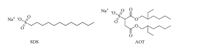
| Scheme 2.Structures of guests SDS and AOT. | |
All solvents and reagents were commercially available used without further purification unless otherwise noted. Anhydrous N,N-dimethylfomamide (DMF) was dried and distilled over calcium hydride (CaH2) under reduced pressure. All aqueous solutions were prepared from distilled water. β-CD of reagent grade (Shanghai Reagent Factory) was recrystallized twice from water and dried in vacuo at 95℃ for 24 h prior to use. 1H NMR and 13C NMR spectra were recorded in D2O on a Bruker AV 400 spectrometer. Mass spectra were performed on a Varian 7.0 T FTICR-MS (MALDI). The elemental analysis (C,H and N) was performed using a Vario EL Cube elemental analyzer (Elementar Ltd. Corp.,Germany). Turbidity measurements were carried out with a Shimadzu UV-3600 spectrophotometer equipped with a PTC-384WI temperature controller. Each sample for TEM measurements was prepared by dropping a 50 μL of sample solution on a copper grid. The grids were then air-dried,and the samples were examined by a Philips EM400st transmission electron microscope. The sample solution for the dynamic light scattering measurements was prepared by filtering the solution through a 450 nm Millipore filter into a clean scintillation vial. The samples were examined by a laser light scattering spectrometer (BI-200SM) equipped with a digital correlator (Turbo Corr.) at 532 nm and a scattering angle of 90°. Per-6-deoxy-6-iodo-β-CD (1) [8] and mono-6-deoxy-6-iodo-β-CD (2) [9] are prepared according to the literature procedure. 2.1. Synthesis of per-6-deoxy-6-(1-methylimidazol-3-ium-3-yl)-b- CD(3)
1 (500 mg,0.26mmol) was dissolved in 1-methylimidazole (3.0 mL,45.0mmol),and the reaction mixture was stirred at 80℃ under argon atmosphere for 48 h. The resultant solutionwas poured into acetone (100 mL). The precipitate formed was collected by filtration and then recrystallized from water to give a translucence flaky solid (355.2mg,55%). 1H NMR (400 MHz,D2O):δ3.30-3.38 (t, 1H,J = 9.2 Hz),3.50-3.56 (d,1H,J = 9.9 Hz),3.75-3.82 (s,3H),3.93- 4.01 (t,1H,J = 9.3 Hz),4.08-4.17 (dd,1H,J = 14.5,5.6 Hz),4.43-4.54 (m,2H),5.02-5.08 (s,1H),7.41-7.45 (s,1H) 7.49-7.53 (s,1H). 13C NMR (100 MHz,D2O):δ36.49,50.05,69.20,71.52,72.04,81.98, 101.92,123.38,124.17,137.46. HRMS (MALDAI,m/z) [M-2I]2+/2 1112.1223,found 1112.1244,[M-3I]3+/3 699.1134,found 699.1126,[M-4I]4+/4 492.6089,found 492.6081,[M-5I]5+/5 368.7062,found 368.7058,[M-7I]2+/7 227.1032,found 227.1032. Elemental Anal. Calcd. for 3: C70H105I7N14O28-8H2O,C 32.05,H 4.65, N 7.48; Found C 32.29,H 4.92,N 7.27. 2.2. Synthesis of mono-6-deoxy-6-(1-methylimidazol-3-ium-3-yl)- β-CD (4)
2 (500 mg,0.40mmol) was dissolved in 1-methylimidazole (2.0 mL,30.0mmol),and the reaction mixture was stirred at 80℃ under argon atmosphere for 24 h. The resultant solutionwas poured into acetone (100 mL). The precipitate formed was collected by filtration and then recrystallized from water to give awhite powder (382.2 mg,72%). 1H NMR (400 MHz,D2O):δ3.21-4.05 (m,45H), 4.93-5.03 (m,7H),7.38-7.42 (s,1H),7.45-7.49 (s,1H). HRMS (MALDAI,m/z) [M-I]+ 1199.4201,found 1199.4203. 3. Results and discussion Possessing the a hydrophilic sulfonate head and one or two hydrophobic tails,SDS and AOT are both able to form selfaggregate in water,and their critical aggregation concentrations (CACs) at 25℃ are 9.7 mmol/L and 2.7 mmol/L,respectively [10]. However,with the addition of host 3,the CAC of guest surfactants greatly decreased,which can be quantitatively investigated by monitoring the dependence of the optical transmittance around 450 nmon the concentration of anionic surfactants in the absence and presence of host 3. In the absence of host 3,the optical transmittance of guest surfactant at 450 nm showed no appreciable changes in the experimental concentration range,indicating that the guest surfactant could not aggregate at these concentrations. As seen in Fig. 1,the optical transmittance of guest surfactant gradually decreased in the presence of host 3,and the host-induced CAC values were measured to be 0.14 mmol/L at 0.04 mmol/L 3 and 0.017 mmol/L at 0.04 mmol/L 3 for SDS and AOT,respectively. Significantly,these CACswere 69 and 158 times lower than the corresponding values of free SDS and AOT, respectively. These results demonstrated that host 3 could induce the aggregation of anionic surfactants at a concentration far lower than its original CAC,especially for the anionic surfactant with a more hydrophobic tail.

|
Download:
|
| Fig. 1.Dependence of the optical transmittance of (a) SDS and (b) AOT at 450 nm in the presence of 0.04 mmol/L 3 at 25℃. | |
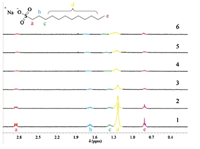
In the molecular aggregate induced by host 3,there exist two possible interactions between host and guest. One is the inclusion complexation of β-CD cavity with guest surfactant,and the other is the electrostatic interactions between seven positively charged methylimidazoliumyl groups and the negatively charged sulfate head of guest surfactant. In order to figure out the contribution of these two interactions,host 4,which also possesses a hydrophobic cavity but only one positively charged methylimidazoliumyl group,was synthesized as a reference compound,and the molecular aggregation behaviours of guest surfactants induced by host 4 were investigated and compared with those induced by host 3. 1H NMR of SDS (1.0 mmol/L) with the increasing concentration of 3 (Fig. 2) showed that the resonance of the Ha atom on SDS was slightly shifted to the higher field,the resonance of the Hb,Hc,Hd and He atoms were shifted to the lower field,and all of the peaks gradually broadened as the concentration of 3 increases. This phenomenon may be attributed to that the electrostatic interactions between imidazolium cations and sulfonate anions decreased the electronegativity of sulfate anions and caused the high field shift of Ha atom,and the low field shifts of Hb,Hc,Hd and He atoms and peaks broadening can be attributed to the Van der Waals effect and the polarity change of ambient environment which was caused by amphiphilic aggregation of SDS. The optical transmittance spectral experiments showed that,in the measured concentration range,the optical transmittance of guest surfactant kept unchanged in the presence of host 4 (Fig. S3 in Supporting information). In addition,as shown in the plot of optical transmittance (Fig. 3) of SDS (0.20 mmol/L) at 450 nm as a function of the concentration of 3 showed that the plot of optical transmittance of SDS the transmittance decreased rapidly and then gradually increased thereafter to a quasi-plateau with increasing the concentration of 3,and the minimum was reached at a SDS/3 ratio of 7,i.e.,a sulfonate anion/imidazolium cation ratio of 1. The rapid decrease of the optical transmittance indicated that a large assembly was formed between 3 and SDS. Then,the addition of an excess amount of 3 led to the formation of a simple 1:1 inclusion complex,resulting in the decrease of turbidity. Similar phenomenon was also observed in the case of 3/AOT system (Fig. 3),and the minimum was also reached at an AOT/3 ratio of 7. In the control experiment,the optical transmittance of SDS or AOT at 450 nmwas nearly unchanged with the addition of 4 (Fig S4 in Supporting information). These results jointly indicated that the electrostatic interactions between imidazolium cations and sulfonate anions, rather than the inclusion complexation of β-CD cavity,are predominant between host and guest.
|
Download:
|
| Fig. 2.Partial 1H NMR spectra of SDS (1.0 mmol/L) with the increasing concentration of 3 in D2O at 25℃. From 1 to 6, [3] =0,1.4×10-5, 1.0×10-4, 1.4×10-4, 2.0×10-4, and 3.3×10-4 mol/L, respectively. | |

|
Download:
|
| Fig. 3.Dependence of the optical transmittance (a) SDS (0.20 mmol/L) and (b) AOT (0.03 mmol/L) at 450 nm with increasing the concentration of 3 at 25℃. | |
The information about the structure and stability of the molecular induced aggregate comes from the Tyndall effect,Zeta-potential,dynamic light scattering (DLS),and transmission electron microscopy. As shown in Fig. 4,the solution of 3/ surfactant exhibits a clear Tyndall effect,indicating the existence of abundant large particles. No Tyndall effect was observed in the solution of free 3 or surfactant,revealing that free 3 and surfactant cannot form nano-scaled aggregates under the same conditions. The Zeta-potential of 3/SDS or 3/AOT ([3]/[surfactant] = 1:7) system was measured to be zero in aqueous solution. This indicated that the charges were packaged inside the aggregate,and the outside surface of the aggregate is the neutral secondary-side of β-CD. The DLS results illustrated the large aggregates of 3/ surfactant with an average diameter of ca. 239 nmfor 3/SDS and ca. 485 nm for 3/AOT,accompanied by a comparatively narrow size distribution. Moreover,no appreciable DLS signals were observed for free 3 and surfactant under the same condition. In the TEM images (Fig. 5),3/SDS showed a number of spherical particles with an average diameter of ca. 100-200 nm,while the average diameter of spherical particles formed by 3/AOT was measured to be ca. 600 nm. The TEM results are consistent with the ones of DLS that host 3 gave the better induced the aggregation effect towards the anionic surfactant with more hydrophobic tail.
The thermal stability of [3]/[surfactant] was measured by the optical transmittance spectra at different temperatures. As shown in Fig. 6,with increasing the temperature from 25℃ to 50℃,the optical transmittance of [3]/[SDS] obviously increased,indicating the disassembly of the induced aggregate. Then,the increased optical transmittance could recover to the original level when decreasing the temperature from 50℃ to 25℃. These cycles could be repeated for several times,demonstrating a good temperatureresponsiveness of the induced aggregate. In contrast,[3]/[AOT] showed the better thermal stability,and the optical transmittance of [3]/[AOT] exhibited very small changes (less than 2%) when switching the temperature between 25℃ and 50℃ (Fig S5 in Supporting information).

|
Download:
|
| Fig. 4.Tyndall effect of 3, SDS, AOT, 3/SDS complex, and 3/AOT in water at 25℃, [3] = 1.4×10-4 mol/L, [SDS] = [AOT] = 1.0×10-3 mol/L. | |

|
Download:
|
| Fig. 5.TEM images of 3/SDS (left) and 3/AOT (right). | |

|
Download:
|
| Fig. 6.(a) Optical transmittance of 3/SDS solution at 450 nm with changing the temperature between 25℃ and 50℃; (b) Dependence of the optical transmittance of 3/SDS solution at 450 nm on temperatures. | |
In summary,a new β-CD derivatives modified with seven positively charged methylimidazoliumyl groups has been successfully synthesized and found able to induced the aggregation of anionic surfactants to form large nanoparticles. Significantly,this host-induced aggregation of surfactants was more effective towards the anionic surfactant possessing more hydrophobic tails, giving the larger diameter,the narrower size distribution and the higher thermal stability of the induced aggregate.
AcknowledgmentsWe thank 973 Programme (No. 2011CB932502) and NNSFC (Nos. 91227107,21432004,and 21272125) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.028.
| [1] | X. Zhang, C. Wang, Supramolecular amphiphiles, Chem. Soc. Rev. 40 (2011) 94– 101. |
| [2] | (a) Y. Wang, N. Ma, Z. Wang, X. Zhang, Photocontrolled reversible supramolecular assemblies of an azobenzene-containing surfactant with a-cyclodextrin, Angew. Chem. Int. Ed. 46 (2007) 2823–2826; (b) H.B. Cheng, H.Y. Zhang, Y. Liu, Dual-stimulus luminescent lanthanide molecular switch based on an unsymmetrical diarylperfluorocyclopentene, J. Am. Chem. Soc. 135 (2013) 10190–10193; (c) K. Wang, D.S. Guo, Y. Liu, Temperature-controlled supramolecular vesicles modulated by p-sulfonatocalix[5]arene with pyrene, Chem.: Eur. J. 16 (2010) 8006–8011; (d) Y.J. Jeon, P.K. Bharadwaj, S. Choi, J.W. Lee, K. Kim, Supramolecular amphiphiles: spontaneous formation of vesicles triggered by formation of a chargetransfer complex in a host, Angew. Chem. Int. Ed. 41 (2002) 4474–4476; (e) Y. Wang, H. Xu, X. Zhang, Tuning the amphiphilicity of building blocks: controlled self-assembly and disassembly for functional supramolecular materials, Adv. Mater. 21 (2009) 2849–2864; (f) Y. Cao, X.Y. Hu, Y. Li, et al., Multistimuli-responsive supramolecular vesicles based on water-soluble pillar[6]arene and SAINT complexation for controllable drug release, J. Am. Chem. Soc. 136 (2014) 10762–10769; (g) D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, Cholinesterase-responsive supramolecular vesicle, J. Am. Chem. Soc. 134 (2012) 10244–10250. |
| [3] | D.S. Guo, Y. Liu, Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications, Acc. Chem. Res. 47 (2014) 1925–1934. |
| [4] | H. Zhang, X. Ma, K.T. Nguyen, Y. Zhao, Biocompatible pillararene-assembly-based carriers for dual bioimaging, ACS Nano 7 (2013) 7853–7863. |
| [5] | (a) U. Rauwald, O.A. Scherman, Supramolecular block copolymers with cucurbit[ 8]uril in water, Angew. Chem. Int. Ed. 47 (2008) 3950–3953; (b) J. Li, L. Zhou, Q. Luo, et al., Cucurbit[7]uril-based vesicles formed by self-assembly of supramolecular amphiphiles, Chin. J. Chem. 30 (2012) 2085– 2090. |
| [6] | (a) R.X. Li, S.M. Liu, J.Q. Zhao, H. Otsuka, A. Takahara, Preparation and characterization of cross-linked β-cyclodextrin polymer/Fe3O4 composite nanoparticles with core-shell structures, Chin. Chem. Lett. 22 (2011) 217–220; (b) L.H. Wang, Z.J. Zhang, H.Y. Zhang, H.L. Wu, Y. Liu, A twin-axial[5]pseudorotaxane based on cucurbit[8]uril and α-cyclodextrin, Chin. Chem. Lett. 24 (2013) 949–952; (c) Z. He, Z. Wang, H. Zhang, et al., Doxycycline and hydroxypropyl-β-cyclodextrin complex in poloxamer thermal sensitive hydrogel for ophthalmic delivery, Acta Pharm. Sin. B 1 (2011) 254–260; (d) S.S. Zhai, Y. Chen, Y. Liu, Selective binding of bile salts by β-cyclodextrin derivatives with appended quinolyl arms, Chin. Chem. Lett. 24 (2013) 442–446. |
| [7] | T. Bojinova, Y. Coppel, N. Lauth-de Viguerie, et al., Complexes between β-cyclodextrin and aliphatic guests as newnoncovalent amphiphiles: formation and physicochemical studies, Langmuir 19 (2003) 5233–5239. |
| [8] | A. Gadelle, J. Defaye, Selective halogenation at primary positions of cyclomaltooligosaccharides and a synthesis of per-3,6-anhydro cyclomaltooligosaccharides, Angew. Chem. Int. Ed. Engl. 30 (1991) 78–80. |
| [9] | B. Brady, N. Lynam, T.O. Sullivan, et al., 6A-O-p-Toluenesulfonyl-β-cyclodextrin, Org. Synth. 10 (2004) 77–79. |
| [10] | (a) J.E. Bujake, E.D. Goddard, Surface composition of sodium lauryl sulphonate and sulphate solutions by foaming and surface tension, Trans. Faraday Soc. 61 (1965) 190–195; (b) H.Z. Yuan, L.F. Shen, Y.R. Du, S. Zhao, J.Y. Yu, Micellization of sodium dodecyl sulfonate and Triton X-100 in polyacrylamide water solution studied by 1H NMR relaxation and two-dimensional nuclear overhauser enhancement spectroscopy, Colloid Polym. Sci. 277 (1999) 1026–1032; (c) A. Chatterjee, S.P. Moulik, S.K. Sanyal, B.K. Mishra, P.M. Puri, Thermodynamics of micelle formation of ionic surfactants: a critical assessment for sodium dodecyl sulfate, cetyl pyridinium chloride and dioctyl sulfosuccinate (Na salt) by microcalorimetric, conductometric, and tensiometric measurements, J, Phys. Chem. B 105 (2001) 12823–12831. |




