Supramolecular approaches have been applied to drug delivery systems [1, 2],and have attractedmuch attention among researchers in various disciplines. Enormous research efforts have beenmade to enhance the therapeutic efficacy and minimize the side effects of anticancer drugs,satisfying the clinical needs. Stimuli-responsive drug delivery systemswith the features of sustained,controlled and targeted drug release are highly desirable [3, 4, 5]. Ideal drug carriers, such as host-guest inclusion complexes [6, 7, 8, 9, 10],micelles [11, 12], hydrogels [13],vesicles [2, 4, 14],liposomes [15, 16],and inorganic nanoparticles [5, 17],should be stable and can avoid nonspecific cell uptake,and beyond that they should also be capable of targeting on tumor sites to realize good treatment efficacy in cancer therapy. Different generations of synthetic macrocycles,i.e.,cyclodextrins, calixarenes (CAs),cucurbiturils,and pillarenes (or pillar[n]arenes), have been employed for the construction of new drug delivery systems [1, 14, 18, 19, 20].
CAs are a family of bowl or cone shaped synthetic supramolecular macrocycles,composed of phenol units linked by methylene bridges through condensation reaction of a phenol and an aldehyde,and they are capable of forming inclusion complexes with suitable guest molecules through the hydrophobic and electron-rich cavity [21]. Significantly,CAs and their derivatives of water-soluble versions in particular,show good biocompatibility and non-cytotoxicity,which are important prerequisites for application in any practical drug delivery systems [22]. In this mini-review,we present a general overview about CA-based drug delivery systems,which can respond to multifarious external stimuli and achieve appreciable therapeutic effect in disease treatment. 2. Inclusion complexes
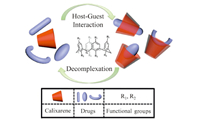
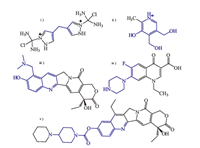
Supramolecular host-guest inclusion complexes were formed by hydrophobic interactions,hydrogen bonding,π-π stacking, charge-transfer,and others,which have provoked lots of interest because of their reversible nature (complexation and decomplexation) [23, 24, 25, 26]. Numerous research groups have reported CAbased drug delivery systems by loading drugs in different inclusion complexation forms (Fig.1). In 2009,Wheate et al. [6] constructed a vehicle for anticancer drug delivery based on p-sulphonatocalix[4]arene (SC[4]A) and dinuclear platinum compound (Fig. 2i) by side-on binding of the two parts. The system would release dinuclear platinum upon in vivo administration,due to high content of blood serum existing in body. In order to amplify the applicability of water-soluble CAs in complex systems,Liu et al. [7, 27] reported the complexation of topotecan and irinotecan (Fig. 2iii and v) with SC[4]A under 1:1 molar ratio,and the inclusion modes were confirmed by means of 1H NMR,DSC,2D NMR and UV-vis spectroscopy. And then Dong et al. [8] constructed the inclusion complex using p-sulphonatocalix[6]arene and vitamin B6 (Fig. 2ii) in acidic and alkaline media. The complex systems could release cargo through disaggregation of the inclusion complexes under certain conditions,which means they can act as an excellent candidate for drug delivery. For the sake of loading larger cargo, such as norfloxacin (Fig. 2iv) and ciprofloxacin,calix[8]arene was chosen as host macrocyclic compound to encapsulate drug into its cavity [9, 28]. Luo et al. [9] reported a smart delivery system,which was composed of p-sulfoniccalix[8]arene and antimicrobial agent in aqueous solutions and could respond to competitive agent,i.e., bovine serum albumin.
|
Download:
|
| Fig. 1.Schematic representation of the formation of inclusion complexes based on CAs and drugs and their decomplexation. | |
|
Download:
|
| Fig. 2.Chemical structures of the studied drugs. The blue parts are the ones that can inset into the cavity of CAs. | |
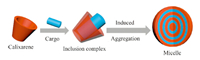
Self-assembly behavior is ubiquitous in living systems. Specific amphiphilic molecules are significant research topics in the fields of materials science and chemical biology because it can be spontaneously developed into micelles (Fig.3),hydrogels and others. In 2011,Zhu et al. [11] took advantage of the host-guest interaction of hydrophilic host molecules,that is,PEGylated calix[4]arene,and hydrophobic chlorin e6 to form supramolecular polymeric micelles,which exhibited more efficient photodynamic therapy efficacy than free chlorin e6. For repairing injury or treating disease in central nervous system (CNS),oxidative stress is a vital element for cell survival. Raston et al. [29] established new micellular systems based on phospholipid calix[4]arenes,which exploited the antioxidants of macrocyclic host compounds sufficiently. Moreover,they can encapsulate fluorescent antioxidant curcumin to realize cargo release. In order to enhance the loading efficiency,Liu et al. [12] made use of drug molecules themselves as an essential unit to form co-assembly nanostructures through simple procedure and subtle design. The amphiphilic assemblies were fabricated via different assembling methods,one was directed by inducing aggregation of antipsychotic drug chlorpromazine with SC[4]A,another co-assembly nanostructure was formed between anionic p-sulfonatocalix[4]arene tetraheptyl ether (SC[4]AH) and cationic chlorpromazine,which exhibited high loading efficiencies (61% and 46%,respectively). In addition,as designed by Xiao et al. [28],the modified amphoteric CAs,with negative and positive charges on each rim,own excellent loading capacity for hydrophobic drug. The amphoteric calix[8]arene can form complexes or multilayers with ciprofloxacin under pH values ranging from 7.05 to 7.58. Furthermore,no matter in acidic medium or basic medium,the assemblies will disaggregate and release drug effectively.
|
Download:
|
| Fig. 3.Schematic illustration of the formation of micelles. | |
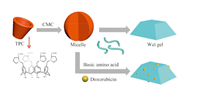
Low molecular weight hydrogels have aroused prodigious attention in many fields. Liu et al. [13] designed a series of supramolecular binary gels by two steps. First,tetra-proline modified calix[4]arenes (TPC) turned to micelles when the concentration of TPC was above the critical micelle concentration (CMC),and then the complexes translated itself into hydrogels with basic amino acids (arginine,histidine and lysine) in acidic conditions. Meanwhile,anticancer drug,doxorubicin hydrochloride (DOX),was entrapped into the gels (Fig.4) and the systems released DOX upon immersion of the hydrogels into water.
|
Download:
|
| Fig. 4.Schematic depiction of gel generation from TPC gelator induced by basic amino acids. | |
Among different forms of nanocarriers for drug delivery and controlled release,lipid vesicles and immune-liposomes exhibited remarkable advantages [2, 15, 30]. In contrast with micelles, vesicles with the hollow cavities prefer to entrap hydrophilic drugs and can capture more. In 2004,Lee et al. [31] constructed the nanocarriers based on CAs backbone,consisting of four hydrophilic dibranched chains with different lengths at the upper border and liphophilic decyl chains at the lower border. Upon increasing the length of hydrophilic chain,large vesicle would transform itself into small spherical micelle. Furthermore,vesicle would also collapse into micelle at acidic conditions thereby realizing cargo release. In 2010,Liu et al. [32] constructed a new nanoscale supramolecular vesicle based on p-sulfonatocalix[5]arene (SC[5]A) and 1-pyrenemethylaminium by host-guest interactions,which can form 1:4 molar ratio of complex and respond to temperature to realize the release of DOX. Schematic illustration of formation of vesicles in response to multi-stimuli to realize drug outflow has been represented in Fig. 5. To better realizing the release diversity, supramolecular binary vesicles based on host-guest complexes of SC[4]A and asymmetric viologen have been built by Liu et al. [4]. Upon increasing temperature,supramolecular interactions between the SC[4]A rings and the organic guests would be weakened,and DOX would be released. Addition of cyclodextrins as the competition reagent or reducing viologen to corresponding neutral molecules or radical cations can also realize that anticancer drug release from the vesicles. Among various kinds of stimuli, enzyme-based systems provide an elegant biocompatible method with high specificity,accuracy and sensitivity for targeting delivery [2, 33]. In 2012,Liu et al. used water-soluble CAs and myristoylcholine to fabricate a new binary vesicle,which can respond to cholinesterase and collapse to choline and myristic acid. Afterwards, they used non-amphiphilic protein and biocompatible SC[4]A to fabricate new nanocarrier by host-guest interactions, possessing the ability to enhance the loading efficiency and minimize the undesired side effects.

|
Download:
|
| Fig. 5.Illustration of formation of vesicles and their response to multi-stimuli to realize drug release. | |
Liposomes and solid lipid nanoparticles (SLNs) as carriers have been used in drug delivery systems and shown good performance owing to their physicochemical properties. In 2003,Shahgaldian et al. [15] used photon correlation spectroscopy (PCS) and atomic force microscopy (AFM) to investigate the new carrier systems, SLNs,which had been fabricated by different amphiphilic calix[4]- arenes and serumalbumin. After adhered by bovine serumalbumin, a layer attain to 17 nmin depthwas generated around the surface of SLNs and no aggregation appeared. Following the principle,they reported protein-modified SLNs by self-assembly interaction of tetrakis(N-methylprolyl)tetraundecylcalix[4]resorcinarene,the systems may open a promising future in drug targeting [34]. In addition,bilayers liposomes based on calix[4]arene and glucose functionalized bolaamphiphile (BA),as the first example with saccharides,were designed by Mancini et al. [16]. Remarkably, because the lipid bilayers possess the BA element,the hydrophilic calcein can effectively entrap into the inner pool. In 2014, Momekova et al. [35] constructed a valid drug delivery [5TD$DIF]system from PEGylated calix[4]arene[3TD$DIF] by heating and solvent evaporation they realized the release of yellow pigment curcumin. 5. Supramolecular nanovalves on mesoporous silica nanoparticles
Over the past couple of decades,mesoporous silica nanoparticles (MSNs) have attracted widespread attention owing to their excellent properties,such as controllable surface functionalization, thermal and photic stability,tunable and uniform pore sizes,and robust storage ability [1, 36]. In addition,good biocompatibility and low cytotoxicity of MSNs made them promising candidate as smart nanocontainers for controlled drug delivery [37]. Choline-SC[4]A [2]pseudorotaxane-based supramolecular nanovalves triggered by enzymes and other stimuli methods were constructed by our research group (Fig. 6) [38]. The nanovalve systems can realize cargo release under 0.3 mg mL-1 of esterase or urease,respectively, through cleaving ester linkage or urea bond sites. Besides,pH and competitive binding agents also led to effective cargo release. On the other hand,light activation is a more promising approach owing to the advantages of remote control and less invasiveness. In particular,compared to UV-vis light [19],near-infrared (NIR) light stimulus[5] enables deeper penetration and less risk of damage to body tissues. Therefore,our group first constructed a novel cancer theranostic hybid platform,based on mesoporous silica-coated gold nanorods (AuNR@MSN) gated by SC[4]A supramolecular switches,for bio-friendly NIR light-triggered cargo release. Mesoporous silica coated on AuNRs guaranteed a high drug payload and easy post-functionalization. Significantly,the plasmonic heating of the NIR light-stimulated AuNR cores can decrease the ring-stalk binding affinity,leading to the dissociation of SC[4]A rings from the stalks on silica surfaces,thus opening the nanovalves and releasing the cargo. Schematic depiction and relevant release profiles under NIR laser with different power densities were given in Fig. 7a and c. The morphology study of AuNR@MSN was done by transmission electron microscope (Fig.7b),which shows nearly monodisperse and uniform structure, intuitively. In addition,our group also reported a smart system, which can release cargo triggered by acetylcholine,a neurotransmitter. This system relies on the different competitive binding strength of macrocyclic receptors with pyridine-modified MSNs and acetylcholine [39].

|
Download:
|
| Fig. 6.Stimuli-responsive supramolecular nanovalves based on MSNs. | |

|
Download:
|
| Fig. 7.Schematic illustration of construction of AuNR@MSN and its NIR lighttriggered cargo release (a), transmission electron microscopic image of AuNR@MSN (b), and release profiles of SC[4]A-capped AuNR@MSN under irradiation with NIR laser of different power densities (c). | |
In this mini-review,we summarized drug delivery systems based on the third generation of supramolecular macrocyclic host compounds,CAs and their derivatives. The most important themes shown here are supramolecular interactions and self-assembly of molecules [40, 41]. Reversible property of supramolecular inclusion complexes,formed via host-guest interactions,made them as a desirable candidate for controlled drug release. Different types of CA-based smart nanocarriers,i.e.,micelles,hydrogels,vesicles, liposomes,and nanovalves,have exhibited excellent properties including stability,sensibility,targeting,and high loading efficiencies. Although scientists have achieved marked success toward CAbased drug delivery systems,so far there are only a few examples implemented on celluar level. We envision that the drug delivery systems based on CAs and their derivatives would have set the stage for biological and clinical testing in the near future.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21272093 and 51473061) and the Fundamental Research Funds for the Central Universities (No. JCKY-QKJC05) for financial support.
| [1] | Y.W. Yang, Towards biocompatible nanovalves based on mesoporous silica nanoparticles, Med. Chem. Commun. 2 (2011) 1033–1049. |
| [2] | D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, Cholinesterase-responsive supramolecular vesicle, J. Am. Chem. Soc. 134 (2012) 10244–10250. |
| [3] | C. Coll, A. Bernardos, R. Martinez-Manez, F. Sancenon, Gated silica mesoporous supports for controlled release and signaling applications, Acc. Chem. Res. 46 (2013) 339–349. |
| [4] | K. Wang, D.S. Guo, X. Wang, Y. Liu, Multistimuli responsive supramolecular vesicles based on the recognition of p-sulfonatocalixarene and its controllable release of doxorubicin, ACS Nano 5 (2011) 2880–2894. |
| [5] | H. Li, L.L. Tan, P. Jia, et al., Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods, Chem. Sci. 5 (2014) 2804–2808. |
| [6] | N.J. Wheate, G.M. Abbott, R.J. Tate, et al., Side-on binding of p-sulphonatocalix[4]- arene to the dinuclear platinum complex trans-[{PtCl(NH3)2}2μ-dpzm]2+ and its implications for anticancer drug delivery, J. Inorg. Biochem. 103 (2009) 448–454. |
| [7] | G.S. Wang, H.Y. Zhang, F. Ding, Y. Liu, Preparation and characterization of inclusion complexes of topotecan with sulfonatocalixarene, J. Incl. Phenom. Macrocycl. Chem. 69 (2011) 85–89. |
| [8] | J. Song, H. Li, J. Chao, et al., Spectroscopic studies on the inclusion interaction of p-sulfonatocalix[6]arene with vitamin B6, J. Incl. Phenom. Macrocycl. Chem. 72 (2012) 389–395. |
| [9] | X. Wang, C. Luo, Z. Lv, F. Lu, Investigation of the inclusion behavior between psulfoniccalix[8]arene and norfloxacin by fluorescence spectroscopy, J. Lumin. 131 (2011) 1986–1990. |
| [10] | D.S. Guo, J. Yang, Y. Liu, Specifically monitoring butyrylcholinesterase by supramolecular tandem assay, Chem. Eur. J. 19 (2013) 8755–8759. |
| [11] | C. Tu, L. Zhu, P. Li, et al., Supramolecular polymeric micelles by the host–guest interaction of star-like calix[4]arene and chlorin e6 for photodynamic therapy, Chem. Commun. 47 (2011) 6063–6065. |
| [12] | Z. Qin, D.S. Guo, X.N. Gao, Y. Liu, Supra-amphiphilic aggregates formed by psulfonatocalix[4]arenes and the antipsychotic drug chlorpromazine, Soft Matter 10 (2014) 2253–2263. |
| [13] | J. Zhang, D.S. Guo, L.H. Wang, et al., Supramolecular binary hydrogels from calixarenes and amino acids and their entrapment-release of model dye molecules, Soft Matter 7 (2011) 1756–1762. |
| [14] | Q. Duan, Y. Cao, Y. Li, et al., pH-Responsive supramolecular vesicles based on water-soluble pillar[6]arene and ferrocene derivative for drug delivery, J. Am. Chem. Soc. 135 (2013) 10542–10549. |
| [15] | J. Gualbert, P. Shahgaldian, A.W. Coleman, Interactions of amphiphilic calix[4]- arene-based solid lipid nanoparticles with bovine serum albumin, Int. J. Pharm. 257 (2003) 69–73. |
| [16] | S. Aleandri, A. Casnati, L. Fantuzzi, et al., Incorporation of a calixarene-based glucose functionalised bolaamphiphile into lipid bilayers for multivalent lectin recognition, Org. Biomol. Chem. 11 (2013) 4811–4817. |
| [17] | Y. Chen, P. Xu, M. Wu, et al., Colloidal RBC-shaped, hydrophilic, and hollow mesoporous carbon nanocapsules for highly efficient biomedical engineering, Adv. Mater. 26 (2014) 4294–4301. |
| [18] | C. Wang, Z. Li, D. Cao, et al., Stimulated release of size-selected cargos in succession from mesoporous silica nanoparticles, Angew. Chem. Int. Ed. 51 (2012) 5460–5465. |
| [19] | Y.L. Sun, B.J. Yang, S.X.A. Zhang, Y.W. Yang, Cucurbit[7]uril pseudorotaxane-based photoresponsive supramolecular nanovalve, Chem. Eur. J. 18 (2012) 9212–9216. |
| [20] | L. Callego-Yerga, M. Lomazzi, F. Sansone, et al., Glycoligand-targeted core–shell nanospheres with tunable drug release profiles from calixarene–cyclodextrin heterodimers, Chem. Commun. 50 (2014) 7440–7443. |
| [21] | V. Bohmer, Calixarenes, macrocycles with (almost) unlimited possibilities, Angew. Chem. Int. Ed. 34 (1995) 713–745. |
| [22] | D.S. Guo, Y. Liu, Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications, Acc. Chem. Res. 47 (2014) 1925–1934. |
| [23] | Y.W. Yang, Y.L. Sun, N. Song, Switchable host–guest systems on surfaces, Acc. Chem. Res. 47 (2014) 1950–1960. |
| [24] | Q. Li, D.S. Guo, H. Qian, Y. Liu, Complexation of p-sulfonatocalixarenes with local anaesthetics guests: binding structures, stabilities, and thermodynamic origins, Eur. J. Org. Chem. (2012) 3962–3971. |
| [25] | X. Zhang, H. Zhao, X. Cao, et al., Hg2+ wettability and fluorescence dual-signal responsive switch based on a cysteine complex of piperidine-calix[4]arene, Org. Biomol. Chem. 11 (2013) 8262–8268. |
| [26] | J.V. Assis, M.G. Teixeira, C.G.P. Soares, et al., Experimental and theoretical NMR determination of isoniazid and sodium p-sulfonatocalix[n]arenes inclusion complexes, Eur. J. Pharm. Sci. 47 (2012) 539–548. |
| [27] | G.S. Wang, H.Y. Zhang, D. Li, et al., Characterisation and antiproliferative activity of irinotecan and sulphonatocalixarene inclusion complex, Supramol. Chem. 23 (2011) 441–446. |
| [28] | Y. Xue, Y. Guan, A. Zheng, H. Xiao, Amphoteric calix[8]arene-based complex for pH-triggered drug delivery, Colloids Surf. B: Biointerfaces 101 (2013) 55–60. |
| [29] | E. James, P.K. Eggers, A.R. Harvey, et al., Antioxidant phospholipid calix[4]arene mimics as micellular delivery systems, Org. Biomol. Chem. 11 (2013) 6108–6112. |
| [30] | M. Ma, P. Xing, S. Li, et al., Advances of host-guest supramolecular vesicles and their properties in drug delivery, Prog. Chem. 26 (2014) 1317–1328. |
| [31] | M. Lee, S.J. Lee, L.H. Jiang, Stimuli-responsive supramolecular nanocapsules from amphiphilic calixarene assembly, J. Am. Chem. Soc. 126 (2004) 12724–12725. |
| [32] | K. Wang, D.S. Guo, Y. Liu, Temperature-controlled supramolecular vesicles modulated by p-sulfonatocalix[5]arene with pyrene, Chem. Eur. J. 16 (2010) 8006– 8011. |
| [33] | K. Wang, D.S. Guo, M.Y. Zhao, Y. Liu, A supramolecular vesicle based on the complexation of p-sulfonatocalixarene with protamine and its trypsin-triggered controllable-release properties,Chem. Eur. J. (2014). |
| [34] | S. Ehrler, U. Pieles, A. Wirth-Heller, P. Shahgaldian, Surface modification of resorcinarene based self-assembled solid lipid nanoparticles for drug targeting, Chem. Commun. (2007) 2605–2607. |
| [35] | E. Drakalska, D. Momekova, Y. Manolova, et al., Hybrid liposomal PEGylated calix[4]arene systems as drug delivery platforms for curcumin, Int. J. Pharm. 472 (2014) 165–174. |
| [36] | J. Liu, W. Bu, L. Pan, J. Shi, NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica, Angew. Chem. Int. Ed. 52 (2013) 4375–4379. |
| [37] | Q.L. Li, Y. Sun, Y.L. Sun, et al., Mesoporous silica nanoparticles coated by layer-bylayer self-assembly using cucurbit[7]uril for in vitro and in vivo anticancer drug release, Chem. Mater. 26 (2014) 6418–6431. |
| [38] | Y.L. Sun, Y. Zhou, Q.L. Li, Y.W. Yang, Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]- arene [2]pseudorotaxanes for controlled cargo release, Chem. Commun. 49 (2013) 9033–9035. |
| [39] | Y. Zhou, L.L. Tan, Q.L. Li, et al., Acetylcholine-triggered cargo release from supramolecular nanovalves based on different macrocyclic receptors, Chem. Eur. J. 20 (2014) 2998–3004. |
| [40] | H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545–552. |
| [41] | L. Wang, L.L. Li, H.L. Ma, H. Wang, Recent advances in biocompatible supramolecular assemblies for biomolecular detection and delivery, Chin. Chem. Lett. 24 (2013) 351–358. |




