b Medical Experiment and Test Center, Capital Medical University, Beijing 100059, China;
c College of Pharmaceutical Sciences, Capital Medical University, Beijing 100059, China
The nitrogen-15 (15N) NMR technique has become one of the most powerful tools in the structural elucidation of nitrogenous organic compounds [1]. Several reports that covered the 15N NMR studies of many nitrogenous organic compounds including alkaloids are available [2, 3]. Due to the low natural abundance of 15N isotope,the 1H-15N correlation spectroscopy,which showed an about 310-fold sensitivity increase over the direct detection method,was employed in the practical applications [1]. By comparison,the 1H-15N HMBC spectra showed 3J long-range correlations from H to N atoμsof the detected molecules,whereas 3J signals from H to C atoμswere observed in the traditional 1H-13C HMBC techniques. In other words,the former may act as a valuable supplement to the conventional NMR experiments in the structural elucidation of nitrogen-containing organic compounds.
Following our previous investigations [4, 5],we studied the 15N NMR of C18-diterpenoid alkaloids,a group of complex and polycyclic diterpenoid alkaloids bearing eighteen carbons and one nitrogen on the skeletons [6]. The present paper describes the long-range 2D 1H-15N NMR spectra data of thirteen C18-diterpenoid alkaloids (Fig. 1),including lappaconitine (1) [7],ranaconitine (2) [7], sinomontanines A (3) and B (4) [8],excelsine (5) [7],8-O-acetylexcelsine (6) [7],sinomontanine H (7) [9],tuguaconitine (8) [10],lappaconidine (9) [7],lappaconine (10) [7],anthriscifolcines E (11),A (12),and C (13) [11]. In addition,the effects of the substituents of nitrogen atoms,the conformations of A ring,as well as protonation,on the nitrogen-15 chemical shifts are observed.
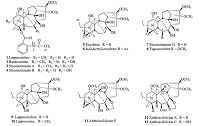
|
Download:
|
| Fig. 1.The structures of compounds 1-13. | |
All NMR spectra were recorded in CDCl3,CD3OD,or acetone-d6 as solvents,using a Bruker Avance III 800 spectrometer operating at frequencies of 800.25 MHz (1H) and 81.1 MHz (15N). The temperature of measurement was 303 K. Sample concentrations ranged from 400 μL to 450 μL in 500 μL deuterium solvents. The 15N chemical shifts were referenced to the signal of 1 mol/L urea in DMSO-d6 (77.0 ppm). The chemical shifts were measured by the HMBC experiments and under identical conditions to those for the described alkaloids.
The pulse conditions were as follows: 90° pulse,duration 8.69 μs for 1H and 35 μs for 15N (5 mm CPQCI 1H/31P/13C/15P/D equipped with a self-shielded z-gradient coil). Computer processing was performed with TOPSPIN Bruker software.
For 1H-15N chemical shift correlations,the HMBC experiments were used. Spectra were acquired as 2048×128 data matrices with 80 transients accumulated per t1 increment with final digital resolution better than 1 and 5 Hz in the 1H and 15N dimensions, respectively. The delay used for evolution of 1H-15N long-range couplings was set to 100 ms in both types of experiments. A gradient pulse duration of 1 ms and a post-gradient recovery delay of 20 μs were used. Gradient ratios for HMBC of 70: 30: 50.1 G cm-1 were applied. The gradient pulses used for HMBC experiments were shaped to a SMSQ10.100 sine envelope and in HMBC experiments square gradients were applied.
2.2. Compounds 1-13All the alkaloids in this study,including lappaconitine (1), ranaconitine (2),sinomontanines A (3) and B (4),excelsine (5),8-O-acetylexcelsine (6),sinomontanine H (7),tuguaconitine (8), lappaconidine (9),lappaconine (10),anthriscifolcines E (11),A (12),and C (13),were isolated by our laboratory from the plants of the genera Aconitum and Delphinium.
3. Results and discussionThe 15N chemical shifts of compounds 1-13 and their trifluoroacetates were listed in Table 1,from which crucial information was observed as below:
|
|
Table 1 15N chemical shifts for compounds 1-13 (15N: 81.1 Hz). |
(1) The 15N1 signal of lappaconitine (1) shifted upfield by 6.2 ppm compared to that of sinomontanine A (3). Apparently,the difference was attributed to the electron-donating effect of ethyl group on N1 atom,indicating that substituents (such as Et group) on nitrogen atomshad a remarkable influence to their chemical shifts. In contrast,the δN values were less affected by the substitution groups on the carbon frameworks,such as C-6 (between compounds 7 and 8),C-8 (between compounds 5 and 6),or C-10 (between compounds 12 and 13),even the presence of 3,4-epoxy units in this class of alkaloids.
(2) The 15N chemical shift data of lappaconitine (1) and ranaconitine (2) were close to each other (δN1~41,δN2~ 130/132), which suggested that the influence of different types of C18- diterpenoid alkaloids (lappaconitine- and ranaconitine-type)was insignificant [6]. Related features were also observed between 8-O-acetylexcelsine (6) and sinomontanine H (7),as well between excelsine (5) and tanguacontine (8). Specifically, the 15N chemical shift values for all the four compounds (5-8) were in the range of δN 50-51 approximately as shownin Table 1.
(3) The δN peak of lappaconidine (9) shifted downfield by about 3.2 ppm by comparison with that of lappaconine (10),which may be ascribed to their different conformations of A ring: boat-form for the former and chair-form for the latter [12]. The same rule was applicable for the other alkaloids with boat conformation of A ring (e.g. sinomontanes A (3) and B (4), excelsine (5),8-O-acetylexcelsine (6),sinomontanine H (7), tuguaconitine (8),and lappaconidine (9) and those with chairform of A ring (e.g. lappaconitine (1),ranaconitine (2), lappaconine (10),and anthriscifolcine E (11). Therefore,the 15N NMR spectra could be employed to predict the conformation of A ring for the C18-diterpenoid alkaloids.
(4) Similar to the 13C NMR,two isomeric δN2 signals for the alkaloids with an N-acetyl anthranilate at C-4 (such as 2 and 4) and their trifluoroacetates were observed in the 15N NMR spectra (Table 1).
(5) As reported in the literature [5],protonation by adding trifluoroacetic acid (TFA) to the free bases revealed a significant downfield shifting effect in the 15N NMR. It is noteworthy that protonation exerts the same influence on the chemical shift values of both nitrogen atoμs(Table 1).
(6) In the 1H-15N HMBC spectra of the alkaloids bearing N-Et groups (compounds 1,2,and 5-13),the 3JN,H long-range correlations between H-22 (δH~ 1.1) and 15N1,and between H-2" (δH~2.2) and 15N2,were the most obvious signals.
4. ConclusionIn conclusion,the 1H-15N HMBC spectra of thirteen C18- diterpenoid alkaloids reported in this paper could be summarized as follows: (1) The 15N chemical shifts were largely affected by the substituents of nitrogen atoμssuch as N-Et,rather than the substitution groups on C-6,C-8,C-10,or the presence of 3,4-epoxy units,or even the types of alkaloids. (2) The corresponding δN values were in the range of δN 44-51 for the alkaloids with boat conformation of A ring (with 1α-OH),while the values were between δN 40-42 for those with chair-form of A ring (with 1α- OCH3). (3) After protonation,the 15N signals for all the compounds (1-13) shifted downfield significantly (δN 13-20 ppm). However, the reason for the unusually strong protonation effect observed for compound 10 (δN 43 ppm) is unclear at the moment. Overall,the application of long-range 2D 1H-15N HMBC experiments serves as a strong complement to the normal NMR techniques in the structural elucidation of nitrogen-containing organic compounds.
AcknowledgmentWe are grateful the National Natural Science Foundation of China (No. 81273387) for the financial support of this research.
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.04. 028.
| [1] | R.M. Silverstein, F.X. Webster, D.J. Kiemle, Spectrometric Identification of Organic Compounds, 7th ed., John Wiley & Sons, New York, 2005, pp. 317-326. |
| [2] | R. Marek, O. Humpa, J. Dostál, J. Slavík, V. Sklenár, 15N NMR study of isoquinoline alkaloids, Magn. Reson. Chem. 37 (1999) 195-202. |
| [3] | G.E. Martin, M. Solntsevan, A.J. Williams, Applications of 15N NMR spectroscopy in alkaloid chemistry, in: E. Fattorusso, O. Taglialatela-Scafati (Eds.), Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH Verlag, Weinheim, 2008, pp. 403-471. |
| [4] | Q.F. Chen, Y.N. Wang, L. Wang, et al., Full assignments of the 1H, 13C and 15N magnetic resonance spectra of two porphyrin compounds, Nat. Prod. Commun. 9 (2014) 547-550. |
| [5] | F.P. Wang, D.L. Chen, H.Y. Deng, et al., Further revisions on the diterpenoid alkaloids reported in a JNP paper (2012, 75, 1145-1159), Tetrahedron 70 (2014) 2582-2590. |
| [6] | F.P. Wang, Q.H. Chen, X.T. Liang, The C18-diterpenoid alkaloids, in: G.A. Cordell (Ed.), The Alkaloids, vol. 67, Elsevier, San Diego, 2009, pp. 1-78. |
| [7] | C.S. Peng, J.Z. Wang, X.X. Jian, F.P. Wang, Alkaloids of Aconitum sinomontanum and Aconitum racemulosum Franch var. pengzhouense, Nat. Prod. Res. Dev. 12 (2000) 45-51. |
| [8] | F.P. Wang, C.S. Peng, X.X. Jian, D.L. Chen, Five new norditerpenoid alkaloids from Aconitum sinomontanum, J. Asian Nat. Prod. Res. 3 (2001) 15-22. |
| [9] | C.S. Peng, D.L. Chen, Q.H. Chen, F.P. Wang, New diterpenoid alkaloids from roots of Aconitum sinomontanum, Chin. J. Org. Chem. 25 (2005) 1235-1239. |
| [10] | Y. He, Studies on the Alkaloidal Components from Delphinium bonvalotii, (M.S. thesis), Sichuan University, Chengdu, 2006. |
| [11] | L. Song,X.X. Liang,D.L. Chen, X.X. Jian,F.P.Wang,New C18-diterpenoid alkaloids from Delphinium anthriscifolium var. savatieri, Chem. Pharm. Bull. 55 (2007) 918-921. |
| [12] | F.P. Wang, Q.H. Chen, The C19-diterpenoid alkaloids, in: G.A. Cordell (Ed.), The Alkaloids: Chemistry and Biology, vol. 69, Elsevier Science, New York, 2010, pp. 1-577. |





