b Department of Solid State Chemistry, Institute of Chemical Technology, Technická 5, 166 28 Prague, Czech Republic;
c Institute of Physics ASCR, v.v.i., Na Slovance 2, 182 21 Praha 8, Czech Republic;
d Department of Chemistry, Faculty of Science, Golestan University, Gorgan, Iran
Azomethines,or Schiff base compounds,are ones of the most widely used organic compounds and played a central role in the development of coordination chemistry of transition metals [1, 2]. In the area of bioinorganic chemistry,interest in Schiff base complexes with transition and inner-transition metals has focused on the role of such complexes in providing useful synthetic models for the metal-containing sites in metallo-proteins and enzymes [3, 4, 5, 6, 7, 8, 9, 10, 11]. In recent years,the coordination chemistry of Schiff base complexes of oxidovanadium(Ⅳ) and dioxidovanadium(V) with N, O,and S-donor chelating ligands have received considerable attention. They show catalytic activity and play vital role in a variety of biochemical processes such as haloperoxidation [12, 13, 14, 15], phosphorylation [16],insulin mimicking [17, 18, 19],nitrogen fixation [20],tumor growth inhibition,and prophylaxis against carcinogenesis [17]. In addition,high-valent vanadium complexes have been considered as new versatile catalytic reagents for a wide range of oxidation reactions [21, 22],like oxidation of olefines and alcohols [23, 24, 25, 26, 27],benzene/alkylaromatic compounds [28, 29],and sulfides [30, 31, 32]. Thus the design of vanadium(Ⅳ) Schiff base complexes containing O,N Schiff base ligands is the center of current interest. Herein we describe the synthesis,characterization, crystal structure determination,thermal study,and catalytic activity of a new oxidovanadium Schiff base complex (VOL) containing tetradentate asymmetric ONN'O' Schiff base ligand (Scheme 1).

|
Download:
|
| Scheme 1.Preparation procedure of the asymmetric teradentate ONN'O' Schiff base ligand (H2L) and its vanadyl complex (VOL). | |
All reagents and solvents for synthesis and analysis were commercially available and purchased from Merck and used as received without further purifications.
2.1. Synthesis of the H2LThis ligand was prepared in two steps: At first,acacen was prepared as described in the literature [33]. A solution of 10 mmol acetylacetone in 50 mL of chloroform was added dropwise to a solution of 10 mmol ethylenediamine in 50 mL of chloroform in a 250 mL round bottom flask at 30 min with stirring at room temperature. After stirring for 3 h,the solvent was evaporated and the resulting solution was reacted with a solution of 10 mmol of 5-bromosalicylaldehyde in 50 mL of methanol and refluxed for 2 h. After evaporation of the solvent,the yellow precipitate was dried in air. Yield (1.98 g,61%). M.p. 77 ℃. Anal. Calcd. for C14H17BrN2O2: C,51.41; H,5.08; N,9.56%. Found: C,51.70; H,5.21; N,9.82%. IR (KBr pellet,cm-1): 1635 and 1613 (s,C = N).
2.2. Synthesis of the VOL1.2 mmol of the H2L was dissolved in a 50 mL of absolute methanol and a solution of 1.2 mmol of VO(acac)2 in 50 mL of absolute methanol was added. The resulting solution was heated for 1 h at 50 ℃. After evaporating the solvent,the green precipitate was dried in air (Yield0.37 g,79%). M.p. 270 ℃. Anal. Calcd. for (C14H15BrN2O2)VO: C,42.79; H,3.36; N,7.03%. Found: C,43.03; H, 3.84; N,7.18%. IR (KBr pellet,cm-1): 1630 and 1605 (s,C = N),974 (s,V = O).
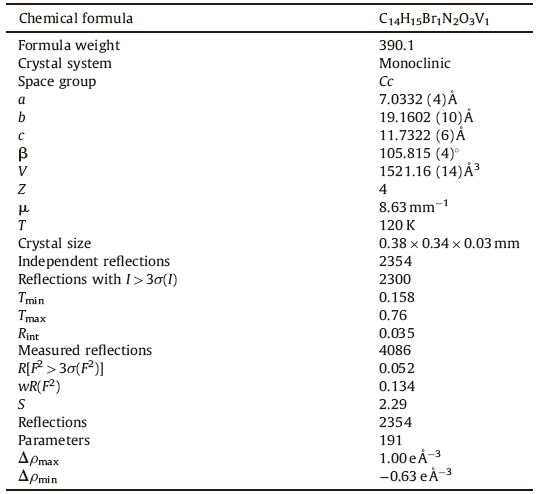
2.3. X-ray crystallographyAsingle crystal of the dimensions 0.38mm× 0.34mm× 0.03mm of VOL was chosen for X-ray diffraction study. Crystallographic measurementswere done at 120 Kwith four circleCCDdiffractometer Gemini of Oxford diffraction,Ltd.,with mirrors-collimated Cu Kα radiation (λ = 1.54184 Å). The crystal structure was solved by charge flipping with the program SUPERFLIP [34] and refined with the Jana2006 program package [35] by full-matrix least-squares technique on F2. The molecular structure plots were prepared by ORTEP Ⅲ [36]. Hydrogen atoms were mostly discernible in difference Fourier maps and could be refined to reasonable geometry. Crystallographic data and details of the data collection,structure solution,and refinements are listed in Table 1.
|
|
Table 1 The crystal data, data collection and structure refinement of VOL. |
The measured sample was a thin slide. However,refinement of the indexed shape indicated that probably only part of this sample was diffracting,because the sample shrank considerably during the refinement. The sample also contained a faint minor domain with the same unit cell parameters but with no systematic orientation with respect to the major domain,which would indicate twinning.
The difference Fourier map contained smeared maxima around positions for Vanadium and Bromine. These maxima could be described by refinement of the 4th order anharmonic ADP for each atom. However,due to insufficient absorption correction, anharmonic ADP refinement may incorrectly identify residues. Keeping in mind the above mentioned limitations of our sample, we decided to refine only harmonic ADP (i.e. ellipsoids).
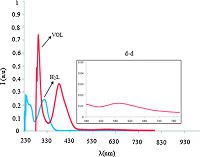
3. Results and discussion 3.1. Synthesis and characterizationThe scheme shows the preparation procedure of the asymmetric tetradentate ONN'O' Schiff base ligand of H2L and its oxidovanadium(Ⅳ) Schiff base complex. Reaction of a methanolic solution of ligand with VO(acac)2 at 50 ℃ for 1 h resulted in production of the asymmetric oxidovanadium(Ⅳ) Schiff base complex (VOL). The Schiff base ligand (H2L) and its VOL complex were characterized by C,H,N analysis,UV-vis,and FT-IR spectra. The chemical composition of the H2L and VOL were determined by the C,H,N analysis and the results confirmed the net chemical compositions. Fig. 1 shows the UV-vis spectra of the H2L and VOL. Three bands were seen in the spectrum of the H2L at 241 nm, 259 nm,and 322 nm. The two bands in the higher energy region (241 nm,259 nm) were attributed to the π→π* transitions and the band at 322 nm was attributed to the π→π* transitions. In the UV-vis spectrum of the VOL complex,these bands shifted to lower energy regions and appeared at 280 nm,292 nm,and 387 nm due to the anionic coordination of the H2L to the vanadium center. In addition,a new band with very low intensity appeared at 604 nm, which was attributed to the d-d transition.
|
Download:
|
| Fig. 1.The UV–vis spectra of the H2L and VOL (10-5 mol/L in CHCl3). | |
In the FT-IR spectrum of H2L,two distinct bands appeared at 1635 cm-1 and 1613 cm-1 due to the two distinct C=N bonds in the H2L,related to the υC=N of salicylate and acetylacetonate moieties,respectively. These bands shifted to the lower wavenumbers in the FT-IR spectrum of the VOL due to the coordination of the nitrogen of imines to the vanadyl center,and appeared at 1630 cm-1 and 1605 cm-1,respectively. The observed band at 974 cm-1 in the FT-IR spectrum of the VOL was attributed to the V=O stretching vibration frequencies in agreement with literature [32, 37, 38],indicating the monomeric five coordination of the VOL as confirmed by crystal structure determination.
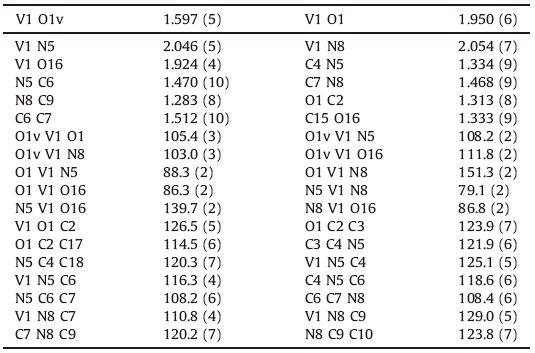
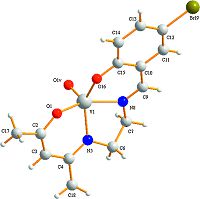
3.2. X-ray structureDetermination of the structure of VOL by single-crystal X-ray crystallography (Fig. 2 and Table 2) shows that the vanadium(Ⅳ) center is coordinated by three oxygen and two nitrogen atoms, resulting in a distorted square pyramid complex. The complex VOL is mononuclear,neutral,and crystallizes in monoclinic space group Cc. The Schiff base ligand acts as a tetradentate ligand through its two iminic nitrogens and two phenolic and acetylacetonate oxygens. The selected bond distances and angles of VOL are listed in Table 2. The V1-N8 and V1-N5 bond distances were found to be 2.054(7) and 2.046(5)Å',respectively,while the V1-O1 and V1-O16 bond distances were found to be 1.950(6) and 1.924(4)Å', respectively,and in agreement with bonds in similar oxidovanadium( Ⅳ) Schiff base complexes [39, 40]. The V1=O1V bond distance (1.597(5)Å') is shorter than the other V-O distances, similarly like other V=O double bonds [39, 40].
|
Download:
|
| Fig. 2.An ORTEP view of VOL showing atoms labeling and ellipsoid probability of 50%. | |
|
|
Table 2 Selected bond distances (Å') and angles (°) of VOL. |
In this structure,the bond angles around vanadium are from 79.1(2)° for N5-V1-N8 to 151.3(2)° for O1-V1-N8,which indicates the significant deformation of the square pyramidal coordination geometry [39, 40].
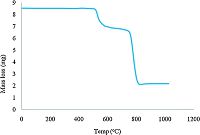
3.3. Thermal studyFig. 3 shows the TG profile of the VOL. The VOL complex was stable up to 280 ℃. After that,it decomposed in two stages by losing Br in the first stage in the temperature range of 301-374 ℃ and losing the residual organic moiety in the second stage in the temperature range of 430-477 ℃,resulting in mixed vanadium oxides. In addition,the VOL complex was taken out in an oven at 660 ℃. The obtained powder from this decomposition was analyzed by XRD-powder diffraction. Based on the resulting XRD pattern,(Fig. 4) the average crystallite size calculated by using Scherrer’s formula was found to be around 49 nm.
|
Download:
|
| Fig. 3.The TGA profile of the VOL. | |

|
Download:
|
| Fig. 4.(a) The XRD pattern of the obtained powder from the thermal decomposition of the VOL complex at 660 ℃ (b) the XRD pattern of the V2O5 from the Merck reference. | |
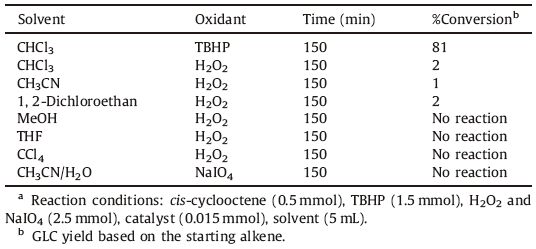
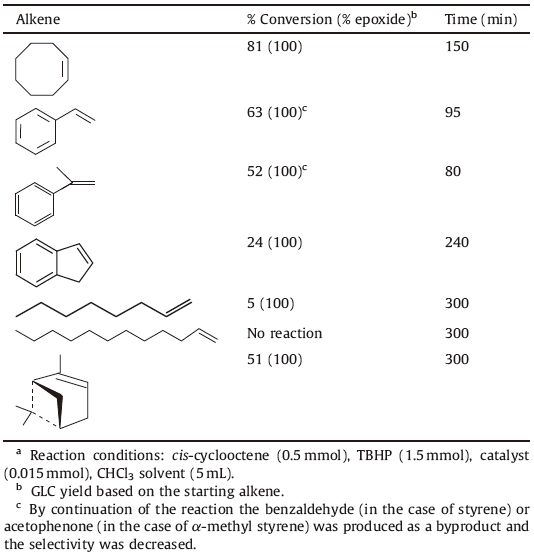
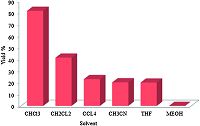
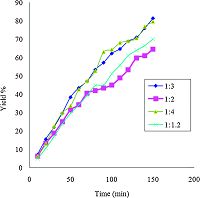
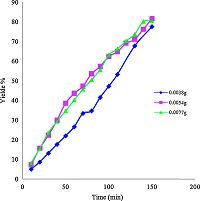
In order to assess the catalytic activity of the VOL complex, cyclooctene was chosen as a model substrate. Different parameters such as solvent,oxidant,and the amount of oxidant and catalyst were optimized in the epoxidation of cyclooctene in the presence of the VOL as a catalyst. The epoxidation of cyclooctene was investigated in the solvents CHCl3,CH2Cl2,CCl4,CH3CN,THF,and MeOH in the presence of tert-butylhydroperoxide (TBHP) as an oxidant and VOL as a catalyst (Fig. 5). High epoxide yields were obtained in the non-coordinating solvents such as CHCl3,CH2Cl2, and CCl4. In the coordinating solvents such as CH3CN,THF,and MeOH,the epoxide yield decreased. Based on the proposed mechanisms,the coordinating solvents compete with TBHP in coordination to the vanadium center and retard the coordination of the TBHP,resulting in decreased epoxide yield [40, 41, 42]. The epoxidation reaction was carried out in three different oxidation media,TBHP,H2O2 and NaIO4,and the results showed that high epoxide yield was obtained in the TBHP (Table 3). The effect of cyclooctene:TBHP ratio on the catalytic epoxidation of cyclooctene was illustrated in Fig. 6. Three ratios (1:2,1:3,and 1:4) were used while the other conditions were kept constant. By increasing the cyclooctene:TBHP ratio from 1:2 to 1:3,the conversion increased slowly,whereas by increasing this ratio from 1:3 to 1:4,the conversion did not change appreciably. The epoxidation reaction was also carried out using three amounts of catalyst (0.01, 0.015 and 0.02 mmol) while keeping other conditions constant (Fig. 7). According to this plot,it is clear that in the case of 0.015 mmol,the catalytic activity of catalyst is similar to the case of 0.02 mmol,but it is higher than in the case of 0.01 mmol. Thus the optimized conditions (0.015 mmol of catalyst,TBHP as an oxidant,1:3 ratio of alkene:TBHP and CHCl3 as a solvent) were chosen,and then the epoxidation of alkenes was tested under these conditions (Table 4). The cyclic alkenes were efficiently converted to corresponding epoxides,but the VOL did not appreciably convert the linear alkenes. In comparison,the catalytic activity of the VOL was lower than the activity of recently reported vanadyl Schiff base complexes.
|
Download:
|
| Fig. 5.The effect of solvent on the conversion of the cyclooctene to cyclooctene epoxide in the presence of TBHP as oxidant by the catalytic amount of VOL in refluxed conditions. | |
|
|
Table 3 The epoxidation of cyclooctene in different oxidation media by the VOL in refluxed conditionsa |
|
Download:
|
| Fig. 6.The effect of alkene/oxidant ratio on the conversion of cyclooctene to cyclooctene epoxide in CHCl3 in the presence of VOL in refluxed conditions. | |
|
Download:
|
| Fig. 7.The effect of catalyst amount on the conversion of the cyclooctene to cyclooctene epoxide in CHCl3 in the presence of TBHP as oxidant and VOL as catalyst in refluxed conditions. | |
|
|
Table 4 The epoxidation of alkenes catalyzed by VOL catalyst with TBHP under reflux conditionsa |
In conclusion,a new asymmetric tetradentate VOL Schiff base complex was synthesized and characterized by C,H,N analysis, FT-IR,and UV-vis spectra. The crystal structure of the VOL was determined by the single crystal X-ray analysis. Thermogravimetric analysis of the VOL showed that it was stable up to 280 ℃,and it decomposed in two stages. Thermal decomposition of the VOL complex at 660 ℃ converts it to V2O5 nanoparticles. In addition,the catalytic activity of the complex was investigated in epoxidation reaction,anddifferent reactionparameterswereoptimized,showing that it can be active and selective under the optimized conditions.
AcknowledgmentsG. Grivani,A. Ghavamiand A.D. Khalaji are grateful to the Damghan University and Golestan University for financial support. Crystallography was supported by the project 14-03276S of the Czech Science Foundation.
Appendix A. Supplementary dataCrystallographic data (excluding structure factors) for the structures reported in this paper have been deposited with the Cambridge Crystallographic Center,CCDC No. 987361. Copies of the data can be obtained free of charge through e-mail (deposit@ ccdc.cam.ac.uk) or web page (http:www.ccdc.cam.ac.uk).
| [1] | M. Shebl, Synthesis and spectroscopic studies of binuclear metal complexes of a tetradentate N2O2 Schiff base ligand derived from 4 6-diacetylresorcinol and benzylamine, Spectrochim. Acta A: Mol. Biomol. Spectrosc. 70 (2008) 850-859. |
| [2] | M. Hobady, T.D. Smith, N,N'-ethylenebis(salicylideneiminato) transition metal ion chelates, Coord. Chem. Rev. 9 (1973) 311-337. |
| [3] | D.N. Dhar, C.L. Taploo, Schiff bases and their applications, J. Sci. Ind. Res. 41 (1982) 501-506. |
| [4] | P. Przybylski, A. Huczynski, K. Pyta, B. Brzezinski, F. Bartl, Biological properties of Schiff bases and azo derivatives of phenols, Curr. Org. Chem. 13 (2009) 124-148. |
| [5] | A.A. Khandar, S.A. Hosseini-Yazdi, S.A. Zarei, Synthesis, characterization and X-ray crystal structures of copper(Ⅱ) and nickel(Ⅱ) complexes with potentially hexadentate Schiff base ligands, Inorg. Chim. Acta 358 (2005) 3211-3217. |
| [6] | P.K. Mascharak, Structural and functional models of nitrile hydratase, Coord. Chem. Rev. 225 (2002) 201-214. |
| [7] | J.G. Muller, L.A. Kayser, S.J. Paikoff, et al., Formation of DNA adducts using nickel(Ⅱ) complexes of redox-active ligands: a comparison of salen and peptide complexes, Coord. Chem. Rev. 185-186 (1999) 761-774. |
| [8] | D.P. Kessissoglou, Homo-and mixed-valence EPR-active trinuclear manganese complexes, Coord. Chem. Rev. 185 (1999) 837-858. |
| [9] | J.W. Pyrz, A.L. Roe, L.J. Stern, L. Que, Model studies of iron-tyrosinate proteins, J. Am. Chem. Soc. 107 (1985) 614-620. |
| [10] | V.E. Kaasjager, L. Puglisi, E. Bouwman, W.L. Driessen, J. Reedijk, Synthesis, characterization and crystal structures of nickel complexes with dissymmetric tetradentate ligands containing a mixed-donor sphere, Inorg. Chim. Acta 310 (2000) 183-190. |
| [11] | A.S. Al-Shihri, Synthesis, characterization and thermal analysis of some new transition metal complexes of a polydentate Schiff base, Spectrochim. Acta A: Mol. Biomol. Spectrosc. 60 (2004) 1189-1192. |
| [12] | A. Butler, J.V. Walker, Marine haloperoxidases, Chem. Rev. 93 (1993) 1937-1944. |
| [13] | M. Andersson, A. Willetts, S. Allenmark, Asymmetric sulfoxidation catalyzed by a vanadium-containing bromoperoxidase, J. Org. Chem. 62 (1997) 8455-8458. |
| [14] | H.B. ten Brink, H.E. Schoemaker, R. Wever, Sulfoxidation mechanism of vanadium bromoperoxidase from Ascophyllumnodosum: evidence for direct oxygen transfer catalysis, Eur. J. Biochem. 268 (2001) 132-138. |
| [15] | V. Trevisan, M. Signoretto, S. Colonna, V. Pironti, G. Strukul, Microencapsulated chloroperoxidase as a recyclable catalyst for the enantioselective oxidation of sulfides with hydrogen peroxid, Angew. Chem. Int. Ed. 43 (2004) 4097-4099. |
| [16] | C.R. Cornman, E.P. Zovinka, M.H. Meixner, Vanadium(Ⅳ) complexes of an active-site peptide of a protein tyrosine phosphatase, Inorg. Chem. 34 (1995) 5099-5100. |
| [17] | P. Noblía, M. Vieites, B.S. Parajón-Costa, et al., Vanadium(V) complexes with salicylaldehyde semicarbazone derivatives bearing in vitro anti-tumor activity toward kidney tumor cells (TK-10): crystal structure of [VVO2(5-bromosalicylaldehyde semicarbazone)], J. Inorg. Biochem. 99 (2005) 443-451. |
| [18] | Y. Shechter, I. Goldwaser, M. Mironchik, M. Fridkin, D. Gefel, Historic perspective and recent developments on the insulin-like actions of vanadium; toward developing vanadium-based drugs for diabetes, Coord. Chem. Rev. 237 (2003) 3-11. |
| [19] | A.M.B. Bastos, J.G. da Silva, P.I.S. Maia, et al., Oxovanadium(Ⅳ) and (V) complexes of acetylpyridine-derived semicarbazones exhibit insulin-like activity, Polyhedron 27 (2008) 1787-1794. |
| [20] | R.R. Eady, Current status of structure function relationships of vanadium nitrogenase, Coord. Chem. Rev. 237 (2003) 23-30. |
| [21] | J.A.L. da Silva, J.J.R. Fraústoda Silva, A.J.L. Pombeiro, Oxovanadium complexes in catalytic oxidations, Coord. Chem. Rev. 255 (2011) 2232-2248. |
| [22] | G. Licini, V. Conte, A. Coletti, M. Mba, C. Zonta, Recent advances in vanadium catalyzed oxygen transfer reactions, Coord. Chem. Rev. 255 (2011) 2345-2357. |
| [23] | V. Conte, F. Di Furia, G. Licini, Liquid phase oxidation reactions by peroxides in the presence of vanadium complexes, Appl. Catal. A: Gen. 157 (1997) 335-361. |
| [24] | S. Mohebbi, D.M. Boghaei, A.H. Sarvestani, Oxovanadium(Ⅳ) complexes as homogeneous catalyst—aerobic epoxidation of olefins, Appl. Catal. A: Gen. 278 (2005) 263-267. |
| [25] | W. Zhang, A. Basak, Y. Kosugi, Y. Hoshino, H. Yamamoto, Enantioselective epoxidation of allylic alcohols by a chiral complex of vanadium: an effective controller system and a rational mechanistic model, Angew. Chem. Int. Ed. Engl. 44 (2005) 4389-4391. |
| [26] | J.H. Hwang, M. Abu-Omar, New vanadium oxazoline catalysts for epoxidation of allylic alcohols, Tetrahedron Lett. 40 (1999) 8313-8316. |
| [27] | M. Bagherzadeh, M. Amini, A new vanadium Schiff base complex as catalyst for oxidation of alcohols, J. Coord. Chem. 63 (2010) 3849-3858. |
| [28] | E. Battistel, R. Tassinari, M. Fornaroli, L. Bonoldi, Oxidation of benzene by molecular oxygen catalysed by vanadium, J. Mol. Catal. A: Chem. 202 (2003) 107-115. |
| [29] | G.B. Shul'pin, G. Süss-Fink, Oxidations by the reagent ‘H2O2-vanadium complex-pyrazine-2-carboxylic acid'. Part 4. Oxidation of alkanes, benzene and alcohols by an adduct of H2O2 with urea, J. Chem. Soc., Perkin Trans. 2 (1995) 1459-1463. |
| [30] | A. Barbarini, R. Maggi, M. Muratori, G. Sartori, R. Sartorio, Enantioselectivesulfoxidation catalyzed by polymer-supported chiral Schiff base-VO(acac)2 complexes, Tetrahedron: Asymmetry 15 (2004) 2467-2473. |
| [31] | T.S. Smith Ⅱ., V.L. Pecoraro, Oxidation of organic sulfides by vanadium haloperoxidase model complexes, Inorg. Chem. 41 (2002) 6754-6760. |
| [32] | R. Ando, H. Ono, T. Yagyu, M. Maeda, Characterization of oxovanadium(Ⅳ)-Schiffbase complexes and those bound on resin, and their use in sulfide oxidation, Inorg. Chim. Acta. 357 (2004) 2237-2244. |
| [33] | A. Biswas, M. Drew, A. Ghosh, Nickel(Ⅱ) and copper(Ⅱ) complexes of unsymmetrical tetradentate reduced Schiff base ligands, Polyhedron 29 (2010) 1029-1034. |
| [34] | L. Palatinus, G. Chapuis, SUPERFLIP-a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions, J. Appl. Crystallogr. 40 (2007) 786-790. |
| [35] | V. Petříček, M. Dušek, L. Palatinus, Crystallographic computing system JANA2006: general features, Zeitschriftfür Kristallographie 229 (2014) 345-352. |
| [36] | L.J. Farrugia, ORTEP-3 for Windows-a version of ORTEP-Ⅲ with a graphical user interface (GUI), J. Appl. Crystallogr. 30 (1997) 565. |
| [37] | R. Ando, S. Mori, M. Hayashi, T. Yagyu, M. Maeda, Structural characterization of pentadentatesalen-type Schiff-base complexes of oxovanadium(Ⅳ) and their use in sulfide oxidation, Inorg. Chim. Acta 357 (2004) 1177-1184. |
| [38] | C.J. Chang, J.A. Labinger, H.B. Gray, Aerobic epoxidation of olefins catalyzed by electronegative vanadyl salen complexes, Inorg. Chem. 36 (1997) 5927-5930. |
| [39] | J. Rahchamani, M. Behzad, A. Bezadpour, et al., Oxidovanadium complexes with tetradentate Schiff bases: synthesis, structural, electrochemical and catalytic studies, Polyhedron 30 (2011) 2611-2618. |
| [40] | S. Rayati, M. Koliaei, F. Ashouri, et al., Oxovanadium(Ⅳ) Schiff base complexes derived from 2 2'-dimethylpropandiamine: a homogeneous catalyst for cyclooctene and styrene oxidation, Appl. Catal. A: Gen. 346 (2008) 65-71. |
| [41] | G. Grivani, S. Delkhosh, K. Fejfarová, M. Dušek, A.D. Khalaji, Polynuclear oxovanadium( Ⅳ) Schiff base complex [VOL2]n (L = (5-bromo-2-hydroxybenzyl-2-furylmethyl) imine): synthesis, characterization, crystal structure, catalytic properties and thermal decomposition into V2O5 nano-particles, Inorg. Chem. Commun. 27 (2013) 82-87. |
| [42] | G. Grivani, G. Bruno, H.A. Rudbari, A.D. Khalaji, P. Pourteimouri, Synthesis, characterization and crystal structure determination of a new oxovanadium(Ⅳ) Schiff base complex: the catalytic activity in the epoxidation of cyclooctene, Inorg. Chem. Commun. 18 (2012) 15-20. |