In 1995,atom transfer radical polymerization (ATRP) using transition-metal complexes as catalyst and organic halide as initiator was almost simultaneously reported by Sawamoto [1] and Matyjaszewski [2] independently. Thereafter,ATRP,as one of the most successful "living"/controlled radical polymerizations,has been widely used to prepare a large variety of well-defined macromolecules including graft [3],block [4, 5, 6],gradient [7],star [8, 9],hyperbranch [10, 11],and dendritic (co)polymers [12],and as well as functional polymeric materials with halogen [13],hydroxyl [14],epoxy [15],cyano [16],and amide groups [17]. Recently,ATRP has attracted extensive attention in industrial applications due to its broad range of applicable monomers,high initiation efficiency, precise control of molecular weights,and mild reaction conditions.
However,ATRP intrinsically suffers from a severe challenge on its road to full commercialization due to the high cost of catalyst loading at industrial scales. In many ATRP reactions,such as those mediated by CuBr/2,20-bipyridine(CuBr/Bpy),CuBr/1,1,4,7,10, 10-hexamethyltriethylene-tetraamine (CuBr/HMTETA) and CuBr/ N,N,N´,N´,N"-pentamethyldiethylenetriamine (CuBr/PMDETA),a nearly equal amount of catalyst and initiator (catalyst/initiator ratio = 1) is usually applied to produce well-controlled polymerizations with appropriate reaction rates. Accordingly,the catalyst concentration in these systems is approximately in the range of 1000-10,000 ppm if the monomer-to-initiator ratio is normally set up between 50 and 500. These catalysts mostly remain in the polymer products after polymerization,consequently coloring, polluting,and poisoning the polymers and deteriorating their properties such as transparence,stability,and dielectricity. Therefore,post-polymerization purification [18, 19] such as extraction,chromatography separation and ion-change as well as in situ catalyst separations (e.g.,liquid-liquid and solid-liquid biphasic polymerization) [20, 21] have been explored to remove the catalyst residue in the polymer products,leading to high costs, environmental problems,and scale-up difficulties.
Many efforts have been made to develop highly active catalytic systems for the purpose of minimizing catalyst consumption and reducing purification costs. Matyjaszewski group reported activators regenerated by electron transfer ATRP (ARGET ATRP) [22] and initiators for continuous activator regeneration ATRP (ICAR ATRP) [23] with catalyst concentrations reduced to tens of ppm.However,these two systems are only applicable to those highly active catalysts such as CuBr/tris[2-(dimethylamino)ethyl]amine (CuBr/Me6TREN) and not suitable for many traditional ATRP catalysts (e.g.,CuBr/Bpy,CuBr/PMDETA and CuBr/HMTETA). Many researchers found that the addition of a small quantity of promoter could improve the catalyst activity and shorten the polymerization time at low catalyst concentrations. For example,Haddleton et al. [24] reported that the ATRP of methyl methacrylate (MMA) catalyzed by CuBr/N-n-butyl-2-pyridylmethanimine was greatly enhanced with the addition of benzoic acid because the benzoic acid coordinated with the copper center. Matyjaszewski et al. found that the addition of copper powder,ascorbic acid,or stannous octoate accelerated the polymerization rates of MMA and styrene (St) due to the reduction of dormant copper(Ⅱ) species to active copper(I) species by the additives [25, 26, 27]. Bai and co-workers [28] demonstrated that certain alcohols were efficient reducing agents for reduction of Cu (Ⅱ) ions to Cu (I) ions in AGET-ATRP of methyl acrylate (MA) and styrene (St). Luo et al. [29] observed a significant enhancement of polymerization rate with the addition of tiny amounts of aluminumhydroxide,boric acid or isobutyl boronic acid in ATRP of St. Interestingly,Zhang et al. [30] found that certain polymerization inhibitors such as 2,4,6-trinitrophenol could speed up the polymerization of MMA catalyzed by FeCl2/PPh3.
We recently reported CuBr/N,N,N´,N´-tetrakis-(2-pyridylmethyl) ethylenediamine (CuBr/TPEN) as a highly active catalyst with triethylamine (TEA) as a promoter for ATRP of MA,MMA and St [31, 32, 33]. The addition of TEA significantly increased the catalytic performance of CuBr/TPEN and consequently the catalyst concentration in these systems was reduced to ppm level. Nevertheless, TEA is a toxic,volatile,and highly flammable liquid with strong pungent odor and harmful irritation to human mucous membranes, skin,and eyes. The addition of TEA as a promoter may bring many adverse consequences (e.g.,bad smell,toxicity and flammability) to polymer products. We herein report a novel non-toxic and biodegradable compound,trolamine,as a highly effective promoter combined with CuBr/TPEN or Me6TREN for ATRP of MA,MMA and St. Based on Regulation (EC) No. 1907/2006 of the European Parliament and of the Council,trolamine is a nonhazardous substance without corrosion and irritation to human skin and has been widely used in cosmetics and medicines. The strong promotion effects of trolamine on the catalytic performance of CuBr/TPEN and CuBr/Me6TREN were investigated,and the kinetics of polymerization as well as the underlying mechanism for the promotion effect are also discussed in this work.
2. Experimental 2.1. MaterialsMethyl acrylate (MA,99%,Aladdin),methyl methacrylate (MMA,99%,Aladdin),styrene (St,99%,Aldrich) were washed with 5% (w/w) NaOH solution,dried over molecular sieve,vacuum distilled,and stored in a refrigerator. Copper(I) bromide (CuBr,98%, Sigma-Aldrich) was purified according to a published procedure [34]. Ligands N,N,N´,N´-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN,98%),tris[2-(dimethylamino)ethyl]amine (Me6TREN,97%) was purchased from Sigma-Aldrich and directly used as received. Ethyl 2-bromoisobutyrate (EBiB,99%),trolamine,tetrahydrofuran (THF) and methanol were from J&K Scientific and used as received without any further treatment.
2.2. Characterization1H NMR spectra were recorded on a Bruker Avance Ⅲ 500 MHz spectrometer using CDCl3as a solvent. Chemical shift δ was given in ppm based on internal standard tetramethylsilane (TMS) (δ = 0 ppm). The monomer conversions of St,MA and MMA were determined using a gravimetric method [35]. The weight-average molecular weight (Mw),number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of poly(methyl methacrylate) (PMMA),poly(methyl acrylate) (PMA),and polystyrene (PS) were detected on a Shimadzu Prominence gel permeation chromatography (GPC) system equipped with a Waters Styragel HR-4E solvent-saving column (molecular weight range: 500- 100,000 g/mol) and a Shimadzu RID-10A refractive index detector. The eluent was THF with a flow rate of 0.3 mL/min at 35 ℃. The GPC calibration curve and universal calibration curve were generated by a series of polystyrene standards (American Polymer Standards Corporation,molecular weight range: 1030-570,000) and used to calculate theMn,Mw andMw/Mn of PS,PMA and PMMA. UV-vis spectra of CuBr2/Me6TREN and CuBr/Me6TREN were measured in methanol with a TU-1900 UV-vis spectrophotometer.
ATRP of MA,MMA and St: A typical procedure for ATRP of MA, MMAor St with CuBr/TPEN as a catalyst was as follows: a magnetic stirring bar and predetermined amounts of TPEN and CuBr were placed in a dry Schlenk tube. Oxygen was removed from the tube by applying high vacuum and backfilling with high purity nitrogen (5 cycles). A certain quantity of degassed monomer (e.g.,MA,MMA or St) was added via a gas-tight syringe under the protection of nitrogen. The tube was transferred to a thermostable oil bath and equilibrated to a desired temperature. Then,required amounts of degassed initiator EBiB and trolamine were added via nitrogenpurged syringes. At timed intervals,samples were withdrawn via degassed gastight syringes,charged into hermetic vials,and stored in a freezer for GPC,1H NMR analysis,and gravimetric measurement. After the polymerization,a certain amount of polymer product was dissolved in THF and precipitated by cold methanol. The precipitates were collected,dried under vacuum at 80 ℃,and characterized by 1H NMR and ICP-MS.
3. Results and discussion 3.1. Promotion effect of trolamine on ATRP of MA catalyzed by CuBr/TPENFor many ATRP catalysts such as CuX/BPY,CuX/PMDETA,and CuX/HMTETA (X = Cl or Br),a reasonable catalyst-to-initiator (catalyst/initiator) ratio of 0.5-1 is generally required to mediate a smooth polymerization. If the catalyst concentration is too low (e.g.,catalyst/initiator <0.1),the polymerization will proceed very slowly and even stop at low conversions. For example,at catalyst/ initiator = 0.01 (CuBr/EBiB = 0.01),the polymerization of MA catalyzed by CuBr/TPEN failed to proceed completely and the monomer conversion stopped at 25% in 3 h (Fig. 1(a),trolamine/CuBr = 0). Nevertheless,at the same catalyst/initiator ratio of 0.01,the addition of 5-fold molar amount of trolamine relative to CuBr (trolamine/ CuBr = 5) significantly promoted the polymerization and increased themonomerconversionupto95.1%in6 hand 94.7%in 2.5 hifmore trolamine was added (trolamine/CuBr = 25). In the presence of trolamine,the ln([M]0/[M]) vs. time plots of the corresponding polymerization were linear,demonstrating that the radical concentration during the polymerization remained essentially constant and the ATRP reaction still kept first-order kinetics with respect to monomer concentration. These results clearly indicated that the addition of trolamine markedly improved the catalytical performance of CuBr/TPENand promoted the polymerization to reach high conversion even at low catalyst concentration.
In the absence of trolamine,the number-average molecular weights of the obtained PMA were very low (Mn < 2000) and the molecular weight distributions were rather broad (Mw/Mn > 2),as shownin Fig. 1(b) (trolamine/CuBr = 0).While in the presence of five times the amount of trolamine relative toCuBr (trolamine/CuBr = 5),the Mn values of prepared PMA increased linearly with monomer conversion and remained close to theoretical values (dashed line) with narrow molecular distributions (Mw/Mn < 1.3). The polymerization at trolamine/CuBr = 25 was also under good control (Mw/ Mn ≤ 1.3). All of these suggest that the addition of trolamine as a promoter significantly enhanced the ATRP of MA catalyzed by CuBr/ TPEN and the catalyst loading could be remarkably reduced by as much as 100 times (from normal CuBr/TPEN = 1 to CuBr/ TPEN = 0.01).

|
Download:
|
| Fig. 1.Effects of trolamine on (a) kinetics and (b) evolution of Mn and Mw/Mn of PMA with monomer conversion in ATRP of MA catalyzed by CuBr/TPEN at CuBr/EBiBr = 0.01. Conditions: 70 ℃,[MA]0 = 11mol/L,[EBiB]0 = 0.11 mol/L,[CuBr/TPEN]0 = 1.1 mmol/L,trolamine/CuBr = 0 (■,□),trolamine/CuBr = 5 (▲,△),and trolamine/CuBr = 25 (●,○). | |
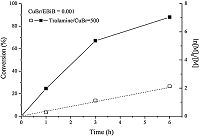
When the catalyst/initiator ratio was further decreased to 0.001 (CuBr/EBiB = 0.001),no polymerization of MA occurred in 6 h without the addition of trolamine. However,the addition of 500- fold molar amounts of trolamine relative to CuBr (trolamine/ CuBr = 500) effectively promoted the polymerization to reach 87% conversion in 6 h,as shown in Fig. 2. The ln([M]0/[M]) vs. time plots were still linear,indicating the polymerization of MA maintained first-order kinetics with respect to monomer concentration. The molecular weight of final PMA measured by GPC (Mn = 8730) was close to its theoretical value (Mn,theo = 7677),and the molecular weight distribution (Mw/Mn) was 1.87.
|
Download:
|
| Fig. 2.Kinetic plots for ATRP of MA catalyzed by CuBr/TPEN at catalyst/ initiator = 0.001 using EBiB as an initiator. Conditions: 70 ℃,[MA]0 = 11 mol/L, [EBiB]0 = 0.11 mol/L,[CuBr/TPEN]0 = 0.11 mmol/L,trolamine/CuBr = 500. | |
Similar significant enhancements of polymerization rate and catalytic performance of CuBr/TPEN were also found in ATRP of MMA and St with the addition of trolamine,as shown in Table 1. Apparently,without the addition of trolamine,the polymerization of MMA catalyzed at catalyst/initiator = 0.01 (CuBr/EBiB = 0.01) only obtained a rather low conversion (6.1%) in 10 h,while the addition of 25-fold molar amount of trolamine relative to CuBr (trolamine/CuBr = 25) remarkably accelerated the polymerization to reach a high conversion of 91.6% in 3 h at the same catalyst-toinitiator ratio of 0.01. The molecular weight of prepared PMMA was close to its theoretical value with a narrow molecular weight distribution (Mw/Mn = 1.34). The ATRP of St in the presence of trolamine achieved 71.4% conversion in 13 h,producing PS with controlled molecular weight and narrow molecular weight distribution (Mw/Mn = 1.38),while the polymerization of St in the absence of trolamine only obtained a very low conversion of 9.3% in 13 h,which indicated that trolamine also had promotion effects on ATRP of MMA and St catalyzed by CuBr/TPEN.
|
|
Table 1 Promotion effects of trolamine on ATRP of MMA and St catalyzed by CuBr/TPEN using EBiB as an initiator. |
CuBr/Me6TREN is an expensive ATRP catalyst and showed very high activity in ICAR ATRP and ARGET ATRP [22, 23, 36]. However,in normal initiation ATRP,CuBr/Me6TREN was found to catalyze the polymerization of MA and St in well-controlled manners at catalyst-to-initiator molar ratios of 0.1 and 0.5 respectively,and failed to polymerize MMA at low catalyst-to-initiator ratios [36]. Therefore,the promotion effect of trolamine on the catalytical performance of CuBr/Me6TREN was examined for ATRP of MA, MMA and St using EBiB as an initiator. As shown in Fig. 3(a) (trolamine/CuBr = 0),the conversion of MA merely reached 15.7% in 10 h without trolamine at a reduced catalyst-to-initiator ratio of 0.01 (CuBr/EBiB = 0.01). But the catalytical performance of CuBr/ Me6TREN was greatly improved in the presence of a small amount of trolamine. The polymerization progressed to much higher conversion of 68.9% in 10 h with the addition of 5 times the molar amount of trolamine relative to CuBr (trolamine/CuBr = 5) and to 94.7% monomer conversion in 6 h at trolamine/CuBr = 25. The ln([M]0/[M]) ~ t plots with the addition of trolamine were linear, implying that the concentration of growing radicals remained essentially constant throughout the polymerizations and the addition of trolamine as a promoter did not change the first-order kinetics of ATRP of MA catalyzed by CuBr/Me6TREN.

|
Download:
|
| Fig. 3.Effects of trolamine on (a) kinetics and (b) evolution of Mn and Mw/Mn of PMA with monomer conversion in ATRP of MA catalyzed by CuBr/Me6TREN at catalyst/ initiator = 0.01. Conditions: 70 8C,[MA]0 = 11 mol/L,[EBiB]0 = 0.11 mol/L,[CuBr/Me6TREN]0 = 1.1 mmol/L,trolamine/CuBr = 0 (■,□),trolamine/CuBr = 5 (●,○),and trolamine/CuBr = 25 (▲,△). | |
In the absence of trolamine,the ATRP of MA at CuBr/EBiB = 0.01 produced PMA with low molecular weights (Mn ≤ 1000) and broad molecular weight distributions (Mw/Mn > 2.5),as shown in Fig. 3(b) (trolamine/CuBr = 0). The polymerization of MA with the addition of trolamine (trolamine/CuBr = 5,trolamine/ CuBr = 25) was well controlled,as evidenced by the linear increase of Mn of PMA against monomer conversion and the relatively low molecular weight distribution (Mw/Mn ~ 1.5). These results demonstrate that trolamine is a versatile and very effective promoter for both CuBr/TPEN and CuBr/Me6TREN.
3.4. Promotion effect of trolamine on ATRP of MMA and St catalyzed by CuBr/Me6TRENThe promotion effect of trolamine was also found in ATRP of MMA and St catalyzed by CuBr/Me6TREN,as shown in Table 2. At CuBr/EBiB = 0.05,CuBr/Me6TREN only mediated the polymerization ofMMA to a low conversion of 16.7% in 6 hwithout trolamine, while the addition of 25-fold molar amount of trolamine relative to CuBr (trolamine/CuBr = 25) promoted the ATRP of MMA to reach 90.1% conversion in 3 h. The number-average molecular weight of obtained PMMA was close to its theoretical valuewith a relatively broad molecular weight distribution (Mw/Mn = 1.41). Similarly,a very low conversion (6.4%) was achieved in the absence of trolamine in ATRP of St at CuBr/EBiB = 0.05,but the addition of trolamine accelerated the polymerization of St to 68.9% conversion in 12 h,producing PS with a molecular weight distribution of 1.38.
|
|
Table 2 Promotion effects of trolamine on ATRP of MMA and St catalyzed by CuBr/Me6TREN using EBiB as an initiator. |
UV-vis spectroscopy was employed to investigate the mechanism of the promotion effect of trolamine. It has been reported that tertiary amines reduced copper(Ⅱ) chloride to copper(I) chloride [37] and that high-valence manganese complexes were reduced to low-valence metal compounds by aliphatic amines [38]. In this work,trolamine was found to reduce CuBr2/Me6TREN to CuBr/ Me6TREN according to their UV-vis spectra,as shown in Fig. 4. Clearly,the spectrumof CuBr2/Me6TREN had an absorption peak at323 nm while the low-valent CuBr/Me6TREN had a broad absorption around 295 nm. With the addition of trolamine to CuBr2/Me6TREN solution,the characteristic peak at 323 nm disappeared and a broad absorption peak similar to the characteristic peak of CuBr/Me6TREN quickly appeared,indicating that CuBr2/Me6TREN had been reduced in situ to CuBr/Me6TREN by trolamine. Therefore,the promotion effect of trolamine could be caused by the reduction of CuBr2/Me6TREN to CuBr/Me6TREN. The addition of trolamine increased the concentration of CuBr/ Me6TREN and meanwhile reduced the concentration of CuBr2/ Me6TREN,resulting in a significant rate increase of the polymerization because the polymerization rate is proportional to the concentration of copper(I) species and inversely proportional to the concentration of copper(Ⅱ) species.

|
Download:
|
| Fig. 4.UV-vis spectra of CuBr2/Me6TREN,CuBr/Me6TREN and CuBr2/ Me6TREN + trolamine. Conditions: 25 ℃,[CuBr2/Me6TREN] = [CuBr/ Me6TREN] = 0.04 mmol/L, [trolamine] = 10 mmol/L. | |
In summary,an effective and versatile promoter,trolamine,has been explored for the ATRP of MA,MMA and St catalyzed by CuBr/ TPEN and CuBr/Me6TREN using EBiB as an initiator. The promoter remarkably improved the catalytical performance of CuBr/TPEN and CuBr/Me6TREN. With the addition of 25-fold molar amount of trolamine relative to CuBr,the catalyst loadings of CuBr/TPEN and CuBr/Me6TREN were dramatically reduced from a catalyst-toinitiator ratio of 1 to 0.01 and 0.05,respectively. The ATRP of MA, MMA and St still showed first-order kinetics in the presence of trolamine and produced PMA,PMMA and PS with controlled molecular weights and narrow molecular weight distributions. The combination of trolamine with low amount of CuBr/TPEN or CuBr/ Me6TREN could enormously reduce the costs of catalyst loading and post-polymerization purification and thus is promising for potential industrial applications.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (No. 21174133) and Zhejiang Science Foundation for Distinguished Young Scholars (No. LR12B04002).
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.012.
| [1] | M. Kato, M. Kamigaito, M. Sawamoto, T. Higashimura, Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris-(triphenylphosphine)ruthenium( Ⅱ)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization,Macromolecules 28 (1995) 1721-1723. |
| [2] | J.S. Wang, K. Matyjaszewski, Controlled/"living" radical polymerization. Atom transfer radical polymerization in the presence of transition-metal complexes, J. Am. Chem. Soc. 117 (1995) 5614-5615. |
| [3] | Y. Shi, Z.F. Fu, Y.D. Zhang, S.K. Jiao, Synthesis of comb like poly(methyl methacrylate) by atom transfer radical polymerization with poly(ethyl 2-bromoacrylate) as macroinitiator, Chin. Chem. Lett. 14 (2003) 1289-1292. |
| [4] | Y. Yi, X.H. Wan, X.H. Fan, R. Dong, Q.F. Zhou, Synthesis of a novel hybrid liquidcrystalline rod-coil diblock copolymer, J. Polym. Sci. Polym. Chem. 41 (2003) 1799-1806. |
| [5] | X.D. Tang, L.C. Gao, X.H. Fan, Q.F. Zhou, Effect of spacer length on the liquid crystalline property of azobenzene-containing ABA-type triblock copolymers via ATRP, Chin. Chem. Lett. 18 (2007) 1129-1132. |
| [6] | X.D. Tang, L.C. Gao, X.H. Fan, Q.F. Zhou, Synthesis and characterization of H-type amphiphilic liquid crystalline block copolymers by ATRP, Chin. Chem. Lett. 19 (2008) 237-240. |
| [7] | K. Matyjaszewski, M.J. Ziegler, S.V. Arehart, D. Greszta, T. Pakula, Gradient copolymers by atom transfer radical copolymerization, J. Phys. Org. Chem. 13 (2000) 775-786. |
| [8] | Y.J. Xu, C.Y. Pan, Block and star-block copolymers by mechanism transformation. 3. S-(PTHF-PSt)4 and S-(PTHF-PSt-PMMA)4 from living CROP to ATRP, Macromolecules 33 (2000) 4750-4756. |
| [9] | X.D. Tang, X.H. Fan, X.F. Chen, Q.F. Zhou, Progress of atom transfer radical polymerization (ATRP) applied to the synthesis of star polymers, Prog. Chem. 17 (2005) 1089-1095 (in Chinese). |
| [10] | S.G. Gaynor, S. Edelman, K. Matyjaszewski, Synthesis of branched and hyperbranched polystyrenes, Macromolecules 29 (1996) 1079-1081. |
| [11] | M.R. Leduc, C.J. Hawker, J. Dao, J.M.J. Fréchet, Dendritic initiators for "living" radical polymerizations: a versatile approach to the synthesis of dendritic-linear block copolymers, J. Am. Chem. Soc. 118 (1996) 11111-11118. |
| [12] | Y.L. Zhao, C.F. Chen, F. Xi, Synthesis of well-defined dendritic-linear diblock and triblock copolymers by controlled free radical polymerization, Chin. Chem. Lett. 13 (2002) 217-218. |
| [13] | C.H. Hu, A.Q. Zhang, Atom transfer radical polymerization of methyl methacrylate initiated by p-chloromethylstyrene copolymers, Fine Chem. 23 (2006) 298-301 (in Chinese). |
| [14] | F. Simal, A. Demonceau, A.F. Noels, Highly efficient ruthenium-based catalytic systems for the controlled free-radical polymerization of vinyl monomers, Angew. Chem. Int. Ed. 38 (1999) 538-540. |
| [15] | K. Matyjaszewski, S. Coca, C.B. Jasieczek, Polymerization of acrylates by atom transfer radical polymerization. Homopolymerization of glycidyl acrylate, Macromol. Chem. Phys. 198 (1997) 4011-4017. |
| [16] | K. Matyjaszewski, S.M. Jo, H.J. Paik, D.A. Shipp, An Investigation into the CuX/2, 2'-Bipyridine (X = Br or Cl) mediated atom transfer radical polymerization of acrylonitrile, Macromolecules 32 (1999) 6431-6438. |
| [17] | X.D. Tang, X.C. Liang, N.F. Han, Y-shaped block copolymers of poly(ethylene glycol) and poly(N-isopropylacrylamide) synthesized by ATRP, Chin. Chem. Lett. 20 (2009) 1353-1356. |
| [18] | K. Matyjaszewski, T. Pintauer, S. Gaynor, Removal of copper-based catalyst in atom transfer radical polymerization using ion exchange resins, Macromolecules 33 (2000) 1476-1478. |
| [19] | Y.Q. Shen, H.D. Tang, S.J. Ding, Catalyst separation in atom transfer radical polymerization, Prog. Polym. Sci. 29 (2004) 1053-1078. |
| [20] | J.H. Xia, T. Johnson, S.G. Gaynor, K. Matyjaszewski, J. De Simone, Atom transfer radical polymerization in supercritical carbon dioxide, Macromolecules 32 (1999) 4802-4805. |
| [21] | J.V. Nguyen, C.W. Jones, Design, behavior, and recycling of silica-supported CuBrbipyridine ATRP catalysts, Macromolecules 37 (2004) 1190-1203. |
| [22] | W. Jakubowski, K. Min, K. Matyjaszewski, Activators regenerated by electron transfer for atom transfer radical polymerization of styrene, Macromolecules 39 (2006) 39-45. |
| [23] | K. Matyjaszewski, W. Jakubowski, K. Min, et al., Diminishing catalyst concentration in atom transfer radical polymerization with reducing agents, Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 15309-15314. |
| [24] | D.M. Haddleton, A.M. Heming, D. Kukulj, D.J. Duncalf, A.J. Shooter, Atom transfer polymerization of methyl methacrylate. Effect of acids and effect with 2-bromo-2-methylpropionic acid initiation, Macromolecules 31 (1998) 2016-2018. |
| [25] | K.Matyjaszewski, S. Coca, S.G. Gaynor,M.L.Wei, B.E.Woodworth, Zerovalentmetals in controlled/"living" radical polymerization,Macromolecules 30 (1997) 7348-7350. |
| [26] | K. Min, H.F. Gao, K. Matyjaszewski, Preparation of homopolymers and block copolymers in miniemulsion by ATRP using activators generated by electron transfer (AGET), J. Am. Chem. Soc. 127 (2005) 3825-3830. |
| [27] | K. Min, W. Jakubowski, K. Matyjaszewski, AGET ATRP in the presence of air in miniemulsion and in bulk, Macromol. Rapid Commun. 27 (2006) 594-598. |
| [28] | Y.X. Wang, X.L. Li, F.F. Du, et al., Use of alcohols as reducing agents for synthesis of well-defined polymers by AGET-ATRP, Chem. Commun. 48 (2012) 2800-2802. |
| [29] | Y.T. Luo, J.M. Zhuang, X.R. Lin, et al., Study of rate-accelerating of aluminum hydroxide, boric acid, and (2-methylpropyl) boronic acid for atom transfer radical polymerization of styrene, J. Xiamen Univ. 47 (2008) 63-66 (in Chinese). |
| [30] | H. Zhang, D.M. Xu, K.D. Zhang, Effect of inhibitors on atom transfer radical polymerization of MMA, Chin. J. Chem. 23 (2005) 913-917. |
| [31] | H.D. Tang, Y.Q. Shen, B.G. Li, M. Radosz, Tertiary amine-enhanced activity of ATRP catalysts CuBr/TPMA and CuBr/Me6TREN, Macromol. Rapid Commun. 29 (2008) 1834-1838. |
| [32] | H.D. Tang, N. Arulsamy, M. Radosz, et al., Highly active copper-based catalyst for atom transfer radical polymerization, J. Am. Chem. Soc. 128 (2006) 16277-16285. |
| [33] | H.D. Tang, M. Radosz, Y.Q. Shen, CuBr2/N,N,N',N'-tetra-[(2-pyridal)methyl] ethylenediamine/tertiary amine as a highly active and versatile catalyst for atomtransfer radical polymerization via activator generated by electron transfer, Macromol. Rapid Commun. 27 (2006) 1127-1131. |
| [34] | J. Queffelec, S.G. Gaynor, K. Matyjaszewski, Optimization of atom transfer radical polymerization using Cu(I)/tris(2-(dimethylamino)ethyl)amine as a catalyst, Macromolecules 33 (2000) 8629-8639. |
| [35] | X. Huang, M.J. Wirth, Surface initiation of living radical polymerization for growth of tethered chains of low polydispersity, Macromolecules 32 (1999) 1694-1696. |
| [36] | J.H. Xia, S.G. Gaynor, K. Matyjaszewski, Controlled/"living" radical polymerization. Atom transfer radical polymerization of acrylates at ambient temperature, Macromolecules 31 (1998) 5958-5959. |
| [37] | J.F. Weiss, G. Tollin, J.T. Yoke, Reactions of triethylamine with copper halides. Ⅱ. Internal oxidation-reduction of dichlorobis(triethylamine)copper(Ⅱ), Inorg. Chem. 3 (1964) 1344-1348. |
| [38] | M.T. Caudle, V.L. Pecoraro, Mechanism for the reduction of the mixed-valent MnⅢMnⅣ[2-OHsalpn]2+ complex by tertiary amines, Inorg. Chem. 39 (2000) 5831-5837. |






