Phosphosilicates exhibit interesting technological and structural properties,which can find applications in fast proton conductors and biomedical materials,such as vehicles for antimicrobial agents,filler and fibers suitable for tissue engineering applications [1, 2, 3, 4]. A special feature of phosphosilicates is the occurrence of hyper-coordinated silicon centers,such as five- or six-coordinated,which is normally dependent on their chemical compositions [5, 6, 7]. For example,six-coordinated silicon has been observed in the glassy matrix of (P2O5)30(SiO2)70 but not in (P2O5)10(SiO2)90 prepared by the same method [8]. Hypercoordinated silicon centers were found to affect not only the backbone conformation but also the physical properties and chemical reactivity of silicon compounds [9, 10],for example,the UV absorption peak of materials with hyper-coordinated silicon centers was found red-shifted relative to their four-coordinated analogs [11].
In liquid solution,a number of organosilicate compounds were found to contain hyper-coordinated silicon centers,which were coordinated with all kinds of atoms,such as O,N,F or Cl [12, 13, 14, 15, 16]. However,silicon atoms of phosphosilicates are most commonly seen as four-coordinated when neighbored with oxygen atoms with only very few exemptions. Besides those phosphosilicates mentioned above,hyper-coordinated silicon centers were also reported to form under high temperature or high pressure process [17, 18, 19, 20, 21, 22]. Organic-inorganic composite or hybrid materials are usually prepared by sol-gel process in situ,which is normally carried out in low temperature and pressure conditions. Therefore varying silicon coordination at relatively low temperature and pressure condition might also be interesting.
It has been appreciated that the relationship between the structure and properties is necessary for the design of materials [8]. The effects of hyper-coordinated silicon centers on the property of phosphosilicate solids are far from fully understood, which is mainly due to the lack of model systems. As discussed above,silicon coordination is usually controlled by changing chemical composition,for example,P/Si ratio [8],thus the effect of silicon coordination becomes hard to be differentiated. Therefore, it is still highly demanding for facile methods to control silicon coordination without changing chemical compositions. Recently, hyper-coordinated silicon species was reported to form in the presence of polyols in aqueous silicate solutions [23]. Thus it was envisaged that polyols may also show similar effect on solid silicates. In the current study,we report our recent progress in synthesizing solid phosphosilicates with hyper-coordinated silicon centers through sol-gel methods in the presence of polyols.
2. Experimental 2.1. MaterialsThe following chemicals were used in the sol-gel preparation without further purification. Myo-inosital (IS),phytic acid (50 wt% aqueous solution) and triethylphosphate (TEP) were purchased from Sigma Aldrich. Xylitol was purchased from TCI. Tetraethyl orthosilicate (TEOS ≥ 99.0%),Ca(NO3)2·4H2O and phosphoric acid were purchased from Sinopharm chemical reagent Co.,Ltd.
Synthesis of calcium phosphosilicate by sol-gel method: The sol-gel prepared materials,(SiO2)24.4(P2O5)53.4(CaO)22.2 and (SiO2)49.3(P2O5)35.9(CaO)14.8,with subscripts representing the oxides molar fractions (mol%),were synthesized using different startingmaterials (Ca(NO3)2·4H2O,TEOS,Phosphoric acid or other phosphorus precursors),with or without polyols. Ca(NO3)2·4H2O was firstlymixedwith ethanol and water at ambient temperature, then TEOS was added through a syringe; the formed solution was stirred magnetically throughout the addition. After 30 min, phosphoric acid (or other phosphorus precursors for example TEP) was added and stirred for an hour. The sols were sealed in polypropylene containers and left to gel. The resultant gels were aged at ambient temperature for 2 days then dried in an oven, firstly at 60 ℃ for 1 week and then at 120 ℃ for another 2 weeks. After that,the gels were further heated to 350 ℃,and then dwelt at this temperature for 1 h. When IS,or xylitol was used,it was mixed with phosphorus acid (or other phosphorus precursors for example TEP),water and ethanol at a given ratio (phosphorus acid/hydroxyl = 1 in molar ratio); the other procedures are the same.
2.2. 31P and 29Si solid state NMRThe magic angle spinning (MAS) solid state nuclear magnetic resonance (NMR) measurements were carried out on the dried materials using an AVANCE III 400 MHz instrument providing 31P and 29Si Larmor frequency of 161.58 MHz and 79.3 MHz, respectively. 4 mm MAS BB probes spinning at 12 kHz were used. For 31P NMR,a 2 μs (~90° tip angle) pulse was used,and the recycle delay was 1 s. For 29Si NMR,a 2.0 μs pulse (~90° tip angle) was used,and the recycle delay was 2 s. 31P and 29Si NMR spectra were referenced using NH4H2PO4 (δP = 0.9 ppm with respect to phosphoric acid) and tetramethylsilane (δSi = 0 ppm),respectively.
2.3. Ion release studiesFor ion release profile determination,the samples were firstly placed in a dialysis bag,then immersed in 50 mL of deionized water (pH 7 ± 1) and incubated in plastic containers at 37 ℃. At various intervals (1 h,3 h,5 h,9 h,24 h,48 h and 120 h,etc.),all the water was taken out of the container for PO43- ion concentration measurements using the Ion Chromatography (IC-883,Metrohm), then 50 mL of fresh deionized water were filled backed into the container for further dissolution. The atomic concentration of silicon was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES: Spectro Arcos Eop,Spectro),which should be mostly in the form of silicate ion due to the aqueous environment.
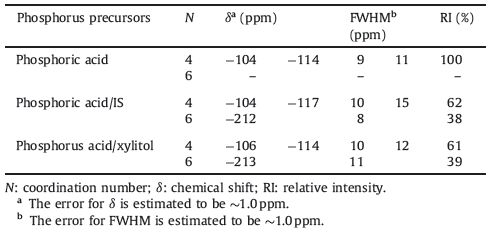
3. Results and discussionTo confirm the structure of the phosphosilicate network,31P MAS solid state NMR was used on the samples obtained from different phosphorus precursors (Fig. 1). Cross-linking in the 31P NMR spectra can be denoted by the Qn notation,where n refers to the number of P-O-X bridges around the phosphate (X = P,Si). As shown in Fig. 1,all the samples treated at 120 ℃ (a-120,b-120, c-120) have similar phosphate network structures,showing an intense and narrow resonance at -1.4 ppm (Q0),which indicates the high degree of mobility for the chemical species giving rise to this resonance. After heat treatment at higher temperature (350 ℃),the 31P resonance of the samples (a-350,b-350,c-350) shows obvious changes,besides the narrow resonance at-1.4 ppm associated with Q0 species,additional resonance appeared at -13.4 ppm and -30.5 ppm,assigned to Q1 and Q3 respectively. Moreover,a resonance is also seen at around -44 ppm,which can be assigned to Q3 phosphates linked to both four- and sixcoordinated silicon atoms through P-O-Si bridges [24]. For the samples b-350 (from phosphoric acid/IS) and c-350 (from phosphoric acid/xylitol),the intensity at this resonance (~-44 ppm) is a little stronger and more negative than the samples a-350 (from phosphoric acid),this difference infers some differences in the number and/or coordination of the silicon neighbors in the P-O-Si bridges [25, 26, 27]. In our previously work,it has been shown that the 31P resonance indeed rely on the silicon content [28].
|
Download:
|
| Fig. 1.The 31P solid state MAS-NMR of (SiO2)24.4(P2O5)53.4(CaO)22.2 using the following precursors after treatment at different temperatures (120 ℃ and 350 ℃): (a) phosphoric acid; (b) phosphoric acid/IS; (c) phosphoric acid/xylitol. | |
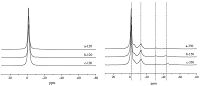
In order to further study the structure of phosphosilicate network,the 29Si MAS-NMR spectra of calcium phosphosilicate xerogels obtained from different phosphorus precursors are shown in Fig. 2 and the results are presented in Table 1. For the samples using phosphoric acid as phosphorus precursor,only signals corresponding to four-coordinated silicon centers are observed at the resonances -104 and -114 ppm (Gaussian fitted peaks, Fig. 2a). The latter is more negative than the Q4-speciation (Si atom coordinated with 4 bridging oxygen atoms at the resonance -104 ppm) in pure silicates,indicating P-O-Si linkages were formed [29, 30, 31],since P is more electronegative than Si. With IS in present,both four- and six-coordinated silicon atoms are observed at -104 ppm,-117 ppm (Gaussian fitted peaks) and -212 ppm, respectively (Fig. 2b),suggesting that IS can help the formation of six-coordinated silicon centers. Another polyol,xylitol,was also used following the same procedure,where six-coordinated silicon centers were also observed (Fig. 2c).

|
Download:
|
| Fig. 2.The 29Si solid state MAS-NMR of (SiO2)24.4(P2O5)53.4(CaO)22.2 using the following precursors after treatment at 350 ℃: (a) phosphoric acid; (b) phosphoric acid/IS; (c) phosphoric acid/xylitol. | |
|
|
Table 1 29Si solid state MAS-NMR spectral parameters obtained from the 29Si spectra in Fig.2 and the associated structural assignments. |
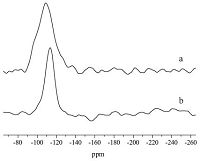
Further experiments were carried out to determine the actual role of polyols. As mentioned earlier,polyols were reported to help the formation of six-coordinated silicon species in aqueous solution [23],which might be key in forming six-coordinated silicon centers in the final calcium phosphosilicate xerogels. However,a control experiment in the absence of phosphorus precursors seemed to rule out this possibility. When no phosphorus precursors were used,no six-coordinated but only fourcoordinated silicon species at -109 ppm were observed (Fig. 3a), suggesting that polyol itself could not lead to the formation of sixcoordinated silicon centers in resultant xerogels but had to collaborate with phosphorus precursors. Heat treatment was also found to be very important. When the resultant xerogels were heat treated at 120 ℃,only four-coordinated silicon species were observed (Fig. 3b),suggesting that the six-coordinated silicon species in solution,if it does exist,are less likely to be transferred into a solid substance straightforwardly.
|
Download:
|
| Fig. 3.The 29Si solid state MAS-NMR of (SiO2)24.4(P2O5)53.4(CaO)22.2 using the following precursors: (a) IS at 350 ℃; (b) phosphoric acid/IS at 120 ℃. | |
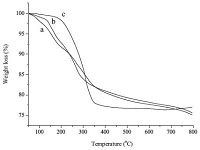
The above observations suggest that both polyols and phosphorus precursors are essential for the formation of hypercoordinated silicon species,and the heat treatment at higher temperature is also necessary,which implies possible reaction between polyol and phosphorus precursor at the elevated temperature. TGA study on these systems showed that there was an obvious weight loss stage at around 170 ℃ for IS and xylitol in the presence of phosphoric acid (Fig. 4a and b),which might be the condensation between phosphoric acid and polyols to form phosphate esters with polyols. When another phosphorus precursor,triethylphosphate (TEP) was used,no such a weight loss was observed,and also no six-coordinated silicon species was observed,presumably due to the lack of trans-esterification reaction between TEP and IS (Fig. 4c).
|
Download:
|
| Fig. 4.The TGA traces of the calcium phosphosilicate xerogels: (a) phosphoric acid/ IS; (b) phosphorus acid/xylitol; (c) TEP/IS. | |
Following the above speculation,phytic acid,the product of esterification between phosphorus acid and IS,was used as the phosphorous precursor to prepare calcium phosphosilicate,which clearly showed the occurrence of six-coordinated silicon centers at -213 ppm (Fig. 5a). In previous studies,it was found that higher phosphate content generally favors the occurrence of sixcoordinated silicon [8]. In this study,for another composition with much lower phosphate content,six-coordinated silicon was also observed when using phytic acid as a precursor,although at a lower relative abundance (Fig. 5b),manifesting the university of this new synthesis route. With extra IS added,no significant changes in the relative abundance of six-coordinated silicon were observed (Fig. 5c),presumably because no more esterification on IS would take place. The above observations further support that the formation of six-coordinated silicon centers may be resulted from the esterification products between polyol and phosphorus precursor,although detailed mechanism is still not clear at this stage.
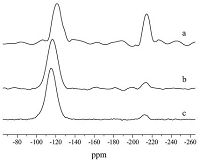
|
Download:
|
| Fig. 5.The 29Si solid state MAS-NMR of samples using the following phosphorus precursors after heated at 350 ℃: (a) phytic acid ((SiO2)24.4(P2O5)53.4(CaO)22.2); (b) phytic acid ((SiO2)49.3(P2O5)35.9(CaO)14.8); (c) phytic acid/IS ((SiO2)49.3(P2O5)35.9 (CaO)14.8). | |
We have successfully demonstrated that polyols can help the formation of six-coordinated silicon centers; however,there was little study on the property of the six-coordinated solid compounds. In samples containing six-coordinated silicon species,the Si-O bond length was calculated to be 1.784Å (Fig. S1 in Supporting information),which was longer than four-coordinated silicon species (1.632Å ) to accommodate more oxygen atoms nearby,and the enthalpy change at 298 K for six-coordinated silicon species was calculated to be 99.6 kJ/mol,which was significantly smaller than that of four-coordinated silicon species (770.6 kJ/mol),thus,one would immediately expect that the Si-O bonds in six-coordinated silicon sites should be more susceptible to hydrolysis. The ion release profiles of the resultant phosphosilicate xerogels were studied upon reacting with pure water. Taking Si atom (as measured by ICP-AES) and PO43- ion as examples,the six-coordinated samples had a significantly higher ion release rate than their four-coordinated counterparts at the same chemical compositions (Fig. 6). The PO43- 3- concentration for six-coordinated sample reached equilibrium of 772 ppm at 49 h (2940 min),which was significantly higher than four-coordinated sample of about 100 ppm at the same time. The Si (most likely in the form of SiO42-) concentration of six-coordinated sample was about 110 ppm at 49 h (equilibrium),also much higher than the comparing sample at the same time. These should make the residual P/Si (by molar) in six-coordinated sample increased dramatically to ~20 at the equilibrium time,much bigger than the original value (~4.38); while in the comparing sample,the residual P/Si decreased to ~3,slightly less than the original value (~4.38). This is rather interesting,since phosphate sites are usually found to be more hydroscopic than silicate sites. This study shows that by simply changing silicon coordination from four- to six-,silicate sites can be made more hydroscopic than phosphate sites. As a consequence,with only 38% of silicon atoms being six-coordinated (Fig. 2b and Table 1),the eventual release of silicate can be enhanced to 90% in 2 days. In previous studies,bioactive silicate glasses have shown great success in many clinical applications especially in dental and orthopedic fields,however,which take SiO2 as a constituent and are rarely absorbed,raising uncertainty about the long-term effects of Si in vivo [32]. Although the dissolution rate can be increased by changing their chemical composition,this nevertheless has the risk of potential chemical hazards. With the control of silicon coordination situation,one may alternatively find another way to adjust silicate dissolution rate,circumventing the problems brought about by changing their chemical compositions.

|
Download:
|
| Fig. 6.Ion release profile for (SiO2)24.4(P2O5)53.4(CaO)22.2 xerogels using the following precursors: (a) phosphoric acid/IS (six-coordinated); (b) phosphoric acid. | |
Polyols can help the formation of six-coordinated silicon in phosphosilicate with only moderate heat treatment (350 ℃) and at ambient pressure. The phosphate esters between phosphoric acid and polyols were found to be essential. The existences of six-coordinated silicon can accelerate the release of silicon significantly,which may have the potential to adjust the degradation rate of silicate compounds for example bioactive glass.
AcknowledgmentsThis work was supported by the State Key Development Program of Basic Research of China (No. 2012CB933200) and National Natural Science Foundation of China (No. 51173193).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.004.
| [1] | M. Nogami, K. Miyamura, Y. Abe, Fast protonic conductors of water-containingP2O5–ZrO2–SiO2 glasses, J. Electrochem. Soc. 144 (1997) 2175–2178. |
| [2] | N.J. Clayden, S. Esposito, P. Pernice, A. Aronne, Solid state 1H NMR study, humiditysensitivity and protonic conduction of gel derived phosphosilicate glasses, J.Mater. Chem. 12 (2002) 3746–3753. |
| [3] | E.A. Abou Neel, D.M. Pickup, S.P. Valappil, R.J. Newport, J.C. Knowles, Bioactivefunctional materials: a perspective on phosphate-based glasses, J. Mater. Chem.19 (2009) 690–701. |
| [4] | L.L. Hench, Bioceramics: from concept to clinic, J. Am. Ceram. Soc. 74 (1991) 1487–1510. |
| [5] | J. Ide, K. Ozutsumi, H. Kageyama, XAFS study of six-coordinated silicon inR2O–SiO2–P2O5 (R = Li, Na, K) glasses, J. Non-Cryst. Solids 353 (2007) 1966–1969. |
| [6] | P. Melnikov, S.B. Santagnelli, F.J. dos Santos, et al., Phosphate functionalization ofspongiolite surface, Mater. Chem. Phys. 82 (2003) 980–983. |
| [7] | R. Dupree, D. Holland, M.G. Mortuza, J.A. Collins, M.W.G. Lockyer, Magic anglespinningNMRof alkali phosphor-alumino-silicate glasses, J. Non-Cryst. Solids 112(1989) 111–119. |
| [8] | N.J. Clayden, S. Esposito, P. Pernice, A.J. Aronne, Solid state 29Si and 31P NMR studyof gel derived phosphosilicate glasses, J. Mater. Chem. 11 (2001) 936–943. |
| [9] | I. EI-Sayed, Y. Hatanaka, S. Onozawa, M.J. Tanaka, Unusual locking of silicon chainsin to all-transoid conformation by pentacoordinate silicon atoms, Am. Chem. Soc.123 (2001) 3597–3598. |
| [10] | I. El-Sayed, Y. Hatanaka, C. Muguruma, et al., Synthesis, X-ray structure, andelectronic properties of oligosilanes containing pentacoordinate silicon moietiesat internal positions, J. Am. Chem. Soc. 121 (1999) 5095–5096. |
| [11] | C. Muguruma, N. Koga, Y. Hatanaka, et al., Theoretical study of ultravioletabsorption spectra of tetra- and pentacoordinate silicon compounds, J. Phys.Chem. A 104 (2000) 4928–4935. |
| [12] | I. Kalikhman, O. Girshberg, L. Lameyer, D. Stalke, D. Kost, Tautomeric equilibriumbetween penta- and hexacoordinate silicon chelates. A chloride bridge betweentwo pentacoordinate silicons, J. Am. Chem. Soc. 123 (2001) 4709–4716. |
| [13] | M. Nakash, M. Goldvaser, Formation of hypervalent complexes of PhCCSiF3 withpyridine through intermolecular silicon nitrogen interaction, J. Am. Chem. Soc.126 (2004) 3436–3437. |
| [14] | I. Kalikhman, B. Gostevskii, O. Girshberg, S. Krivonos, D. Kost, Donor-stabilizedsilyl cations 4: N-isopropylidene hydrazides, novel bidentate ligands for pentaandhexacoordinate silicon chelates, Organometallics 21 (2002) 2551–2554. |
| [15] | N. Kano, F. Komatsu, M. Yamamura, T. Kawashima, Reversible photoswitchingof the coordination numbers of silicon in organosilicon compounds bearing a2-(phenylazo) phenyl group, J. Am. Chem. Soc. 128 (2006) 7097–7109. |
| [16] | J.B. Lambert, S.R. Singer, Self-assembled macrocycles with pentavalent siliconlinkages, J. Organomet. Chem. 689 (2004) 2293–2302. |
| [17] | G. Serghiou, R. Boehler, A. Chopelas, Reversible coordination changes in crystallinesilicates at high pressure and ambient temperature, J. Phys. Condens. Matter12 (2000) 849–857. |
| [18] | M. Nogami, K. Miyamura, Y. Kawasaki, Y. Abe, Six-coordinated silicon in SrO–P2O5–SiO2 glasses, J. Non-Cryst. Solids 211 (1997) 208–213. |
| [19] | T.L. Weeding, B.H.W.S.W. de Jong, S. Veeman, B.G. Aitken, Silicon coordinationchanges from 4-fold to 6-fold on devitrification of silicon phosphate glass, Nature318 (1985) 352–353. |
| [20] | E. Ohtani, F. Taulelle, C.A. Angell, Al3+ coordination changes in liquid aluminosilicatesunder pressure, Nature 314 (1985) 78–81. |
| [21] | X. Xue, J.F. Stebbins, M. Kanzaki, R.G. Tronnes, Silicon coordination and speciationchanges in a silicate liquid at high pressures, Science 245 (1989) 962–964. |
| [22] | J.F. Stebbins, M. Kanzaki, Local structure and chemical shifts for six-coordinatedsilicon in high-pressure mantle phases, Science 251 (1991) 294–298. |
| [23] | S.D. Kinrade, J.W.D. Nin, A.S. Schach, et al., Stable five- and six-coordinated silicateanions in aqueous solution, Science 285 (1999) 1542–1545. |
| [24] | P. Hartmann, C. Jana, J. Vogel, C. Jager, P-31 MAS and 2D exchange NMR ofcrystalline silicon phosphates, Chem. Phys. Lett. 258 (1996) 107–112. |
| [25] | D. Miyabe, M. Takahashi, Y. Tokuda, T. Yoko, T. Uchino, Structure and formationmechanism of six-fold coordinated silicon in phosphosilicate glasses, Phys. Rev. B71 (2005) [5TD$DIF]172202. |
| [26] | C. Coelho, F. Babonneau, T. Azaïs, et al., Chemical bonding in silicophosphate gels:contribution of dipolar and J-derived solid state NMR techniques, J. Sol–Gel. Sci.Technol. 40 (2006) 181–189. |
| [27] | S.P. Szu, L.C. Klein, M. Greenblatt, Effect of precursors on the structure ofphosphosilicate gels-Si-29 and P-31 MAS NMR-study, J. Non-Cryst. Solids 143(1992) 21–30. |
| [28] | A. Li, D. Wang, J. Xiang, et al., Insights into new calcium phosphosilicate xerogelsusing an advanced characterization methodology, J. Non-Cryst. Solids 357 (2011)3548–3555. |
| [29] | R. Dupree, D. Holland, M.G. Mortuza, 6-Coordinated silicon in glasses, Nature 328(1987) 416–417. |
| [30] | S. Prabakar, K.J. Rao, C.N.R. Rao, A MAS NMR investigation of lead phosphosilicateglasses: the nature of the highly deshielded six-coordinated silicon, Mater. Res.Bull. 26 (1991) 285–294. |
| [31] | M.G. Mortuza, M.R. Ahsan, J.A. Chudek, G. Hunter, First evidence for the coexistenceof four-, five- and six-coordinated silicon in glasses prepared at ambientpressure, Chem. Commun. (2000) 2055-2056. |
| [32] | V. Salih, K. Franks, M. James, G.W. Hastings, J.C. Knowles, Development of soluble glasses for biomedical use: Part II. The biological response of human osteoblast cell lines to phosphate-based soluble glasses, J. Mater. Sci.: Mater. Med. 11 (2000) 615-620. |