b Medical School of Nanjing University, Nanjing 210093, China
Nitric oxide (NO) is a multifunctional molecular involved in a variety of physiological and pathological processes. Generated by nitric oxide synthase (NOS) from oxidation of L-arginine,NO binds to its primary receptor,the soluble guanylylcyclase,leading to the synthesis of the signal transducer cGMP [1, 2]. Also,NO could be produced from nitrogen oxides such as nitrite and nitrate,which can serve as storage pools for NO when enzymatic activity of NOS is suppressed [3]. Besides the wide implications of NO derivatives in the treatment of vascular disorders [4],NO level or NOS activity was observed to be correlated with clinical tumorigenesis [5, 6, 7, 8]. Particularly,cell survival signaling is significantly affected by the level of NO. Generally,NO is considered to promote tumor angiogenesis at low concentration,however,it contributes to apoptosis in tumor cells at high concentration [9, 10, 11].
Given the fact of the high-active property of NO,NO donors, including organic nitrates,furoxans,metal-NO complexes,Snitrosothiols, sydnonimines,diazeniumdiolates (NONOates),and NO-drug hybrids,with the controllable NO-releasing property are attractive substitutes for NO [12]. More specifically,conjugation of NO donors to tissue targeting compounds could achieve organdelivery effect. In practical application for anticancer research,it is more feasible to generate novel NO-drug hybrids by linking various NO donors to a parent compound (with anti-tumor activity) to display the synergistic anti-tumor activity.
Betulinic acid (BA) is a natural pentacyclic triterpene with antitumor [13] and anti-HIV activities [14, 15],and so on. It was reported that BA exhibited selective toxicity to varieties of tumor cells but not normal tissues,which can be an ideal lead compound for anticancer treatment [16]. In the present study,based on the principle of pro-drug,we designed a series of novel NO-BA hybrids which 3-hydroxyl group and 28-carboxyl group of BA were chosen to be coupled with NO donors,nitrate and furoxan,respectively. Considering that increasing the electron density in A-ring of BA can improve its anti-tumor activity,therefore,we selected an oxime group in C-3 position of BA to be a parent compound,along with 3- O-derivatives. In addition to investigating the effect of different length and structure of the linker which attaches BA and NO donor, we also considered the fact that tumor cells display the character of “addicted nitrogen” and increase the transfer rate of amino acids, as a result,we supposed that introducing amino acids into the target compounds may enhance their selectivity to tumor cells. Consequently,we introduce amino acids into the linker of BA and furoxans.
Totally,13 NO-BA hybrids were successfully synthesized,with their structures characterized by IR,1H NMR and MS. The in vitro anti-tumor activities were determined in mouse melanoma B16 cells and hepatocellular carcinoma HepG2 cells,respectively. Importantly,compounds 11a and 11b showed significantly stronger cytotoxicity against both B16 cells and HepG2 cells than BA itself. Our data suggested that coupling NO donors to BA is a promising approach to generate highly active anti-tumor compounds.
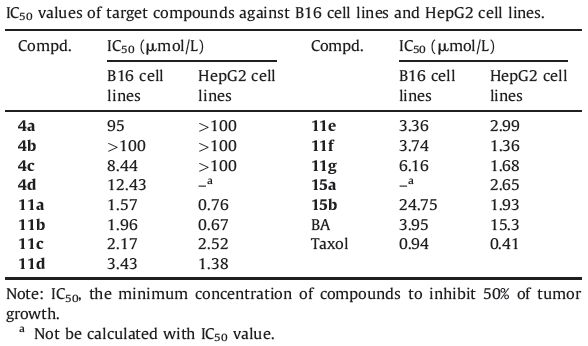
2. ExperimentalThe general route for BA derivatives coupled with nitrates is outlined in Scheme 1. BA was first oxidized to 1 by Jones’ reagent, then,reacted with dibromoalkane in the presence of K2CO3 in DMF to give the corresponding C-28 esters (2a-d). 3a-d were obtained from the reaction of 2a-d and AgNO3 in the dark. Subsequently, 3a-d and hydroxylamine hydrochloride were dissolved in pyridine. The solution was stirred for 24 h at 50 ℃,giving the target compounds 4a-d.
|
Download:
|
| Scheme 1.Synthesis of compounds 1,2,3 series. Reagents and conditions: (i) Jones,0 ℃; (ii) Br(CH2)nBr; (iii) AgNO3; (iv) NH2OH·HCl. | |
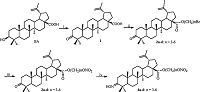
Considering the less reactivity of C-28 carboxyl,oxalyl chloride was used to activate BA,giving compound 10. Then,the reaction of 10 and furoxan intermediates 9a-g led to the target compounds 11a-g. The general route is outlined in Scheme 2,among which 6a-g were derived from 5 (obtained as previously described) [17] and the corresponding diols. Subsequently,6a-g reacted with glycine protected by di-tert-butyl dicarbonate (Boc2O),giving 8a-g. Then, 8a-g were treated with trifluoroacetic acid (TFA),affording 9a-g.
|
Download:
|
| Scheme 2.Synthesis of compounds 6,8,9,10,11 series. Reagents and conditions: (i) HO-R-OH,NaOH; (ii) Boc2O; (iii) DCC/DMAP; (iv) TFA; (v) oxalyl chloride; (vi) Et3N. | |
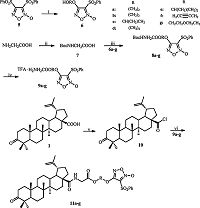
15a and 15b,furoxan-based derivatives of BA at C-3 position, were also synthesized in this study. The general route is outlined in Scheme 3. BA was treated with succinic anhydride to prepare compound 12,then,reacted with 6b to get 13. Subsequently,13 reacted with 14a or 14b respectively in the presence of 4- dimethylamino pyridine (DMAP) and 1-ethyl-(3-dimethylaminopropyl)- carbodiimide hydrochloride (EDCI) to generate target compounds 15a or 15b.
|
Download:
|
| Scheme 3.Synthesis of compounds 12,13,15 series. Reagents and conditions: (i) succinic anhydride; (ii) EDCI/DMAP; (iii) DCC/DMAP. | |
The anti-tumor bioactivities of these compounds were determined in vitro by MTT assay against HepG2 and B16 cells. Taxol was selected as positive control. The data were calculated and presented as IC50 values in Table 1. We observed that the majority of NO-BA hybrids were bioactive with growth inhibitory effect on both HepG2 cells and B16 cells. Among them,most nitrate-based derivatives of BA lost their anti-tumor activity compared with BA and the positive control taxol,which is in consistent with our previous report on NO-pentacyclic triterpene hybrids. Herein,we speculate that the NO releasing kinetics from nitrate donors might result in a low concentration,which in turn protects the tumor cell from apoptosis.
|
|
Table 1 IC50 values of target compounds against B16 cell lines and HepG2 cell lines. |
As shown in Table 1,most furoxan-based derivatives of BA displayed higher cytotoxicity than BA,while only compound 11g exhibited slightly weaker potency against B16 cells. And the most promising candidates as potential alternative drugs were 11a and 11b,which showed comparable cytotoxicity against both HepG2 and B16 cells to the positive control (taxol). This preliminary result indicates that oxygen in the linker (11g) cannot potentially increase the antitumor activity of BA. The antitumor activity of compounds with branched linker are inferior to those with straight linker with the same number of carbons,which can be explained by the fact that 11c and 11e are less potent than 11b and 11d, respectively. Considering the overall anti-tumor activity at different concentrations,to prolong the length of the linker failed in improving the anti-tumor activity of these derivatives against B16 cells,which can be interpreted by the fact that 11a,11b,and 11d weakened the potency in turn. However,it did not show obvious structure and activity relationships (SARs) among these derivatives against HepG2 cells. Additionally,the triple bond derivative 11f can slightly enhance the cytotoxicity only at high concentration. Further SARs are required to be certified in this series.
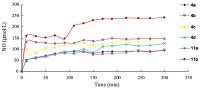
In order to identify whether the amount of NO released by these NO-BA hybrids is the major impact factor of the anti-tumor activity,we measured the in vitro NO-releasing amount of these BA derivatives by Griess method. As shown in Fig. 1,the amount of NO released by the furoxan-based derivatives is generally higher than that of nitrate-based derivatives,especially some furoxan-based derivatives,such as 11b (242 μmol/L) releases more than twofold of NO compared with 4a (94 μmol/L) and 4b (95 μmol/L),which is consistent with the anti-tumor activity of the corresponding derivatives. Thus,furoxan-based derivatives possessing high cytotoxicity can be partially explained by the fact that higher level of NO contributes to the anti-tumor activity.
|
Download:
|
| Fig. 1.NO2- concentrations which represent the quantity of NO were determined by Griess assay in vitro. Griess reagent could combine with NO2- and form the chromophore after 10 min at 37 ℃,the absorbance then was measured at 540 nm. | |
Taking the above results into account,we synthesized new furoxan-based derivatives modified at C-3 position of BA. Unfortunately,both 15a and 15b resulted in poor antitumor activity. Especially,the antitumor activity of 15b (furoxan-based derivative at C-3 position) was obviously inferior to that of 11a-g (furoxan-based derivatives at C-28 position) against B16 cells. Thus,we conservatively speculated that C-28 position of BA was favorable to be modified with furoxan,which may afford more active compounds with anti-tumor activity.
4. ConclusionIn summary,we have designed and synthesized thirteen novel NO-releasing derivatives of BA coupled with nitrates and furoxans. The anti-tumor bioactivities of these derivatives were determined by the MTT assay in vitro against HepG2 and B16 cells. Nitratebased derivatives of BA resulted in poor activity against both HepG2 cells and B16 cells,while the majority of furoxan-based derivatives enhanced the anti-tumor activity of BA,which consists with our hypothesis. Subsequently,we detected the NO-releasing amount of these derivatives to investigate whether it affects the antitumor activity of these derivatives. As a result,furoxan-based derivatives indeed released larger among of NO than that of nitrate derivatives,which may partly explain the higher antitumor activity of furoxan-based derivatives—high concentration of NO contribute to the antitumor activity. Among all these BA derivatives,compound 11a and 11b showed promising antitumor activity (IC50<1 μmol/L),which were comparable to that of the positive control. Further research is ongoing to illustrate more SARs,and we believe our finding will be of value for further utilization of BA derivatives in the therapeutic interventions of some cancers.
AcknowledgmentThis work was financially supported by the National Natural Science Foundation of China (No. 20972190),and the Academic Leadership of ‘QingLan’ Project of Jiangsu Department of Education.
Appendix A. Supporting InformationSupporting Information associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet. 2015.04.002.
| [1] | S. Moncada, Nitric oxide: discovery and impact on clinical medicine, J. R. Soc. Med. 92 (1999) 164-169. |
| [2] | P.J. Andrew, B. Mayer, Enzymatic function of nitric oxide synthases, Cardiovasc. Res. 43 (1999) 521-531. |
| [3] | J.O. Lundberg, E. Weitzberg, NO generation from nitrite and its role in vascular control, Arterioscler. Thromb. Vasc. Biol. 25 (2005) 915-922. |
| [4] | R.M. Touyz, E.L. Schiffrin, Reactive oxygen species in vascular biology: implications in hypertension, Histochem. Cell Biol. 122 (2004) 339-352. |
| [5] | J.E. Albina, J.S. Reichner, Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis, Cancer Metastasis Rev. 17 (1998) 39-53. |
| [6] | F. Dodd, M. Limoges, R.T.M. Boudreau, et al., L-Arginine inhibits apoptosis via a NO-dependent mechanism in Nb2 lymphoma cells, J. Cell. Biochem. 77 (2000) 624-634. |
| [7] | L.A. Ridnour, K.M. Barasch, A.N. Windhausen, et al., Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation, PLoS ONE 7 (2012) e44081. |
| [8] | V. Umansky, V. Schirrmacher, Nitric oxide-induced apoptosis in tumor cells, Adv. Cancer Res. 82 (2001) 107-131. |
| [9] | D.D. Thomas, M.G. Espey, L.A. Ridnour, et al., Hypoxic inducible factor 1a, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide, Proc. Natl. Acad. Sci. U. S. A. 101 (2004) 8894-8899. |
| [10] | L.A. Ridnour, D.D. Thomas, C. Switzer, et al., Molecular mechanisms for discrete nitric oxide levels in cancer, Nitric Oxide 19 (2008) 73-76. |
| [11] | S. Mocellin, V. Bronte, D. Nitti, Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities, Med. Res. Rev. 27 (2007) 317-352. |
| [12] | S. Korde Choudhari, G. Sridharan, A. Gadbail, V. Poornima, Nitric oxide and oral cancer: a review, Oral Oncol. 48 (2012) 475-483. |
| [13] | S.Y. Ryu, S.U. Choi, S.H. Lee, et al., Antitumor triterpenes from medicinal plants, Arch. Pharm. Res. 17 (1994) 375-377. |
| [14] | Y. Kashiwada, F. Hashimoto, L.M. Cosentino, et al., Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents, J. Med. Chem. 39 (1996) 1016-1017. |
| [15] | F. Li, R. Goila-Gaur, K. Salzwedel, et al., PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing, Proc. Natl. Acad. Sci. U. S. A. 100 (2003) 13555-13560. |
| [16] | E. Pisha, H. Chai, I.S. Lee, et al., Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis, Nat. Med. 1 (1995) 1046-1051. |
| [17] | L. Fang, Y.H. Zhang, J. Lehmann, et al., Design and synthesis of furoxan-based nitric oxide-releasing glucocorticoid derivatives with potent anti-inflammatory activity and improved safety, Bioorg. Med. Chem. Lett. 17 (2007) 1062-1066. |