University, Zhengzhou 450052, China;
b Department of Chemistry, College of Chemistry and Chemical Engineering, The Key Laboratory for Chemical Biology of Fujian Province, Xiamen University, Xiamen 361005, China
It is well known that fused pyridines and thienopyrimidines have many valuable pharmacological properties,such as antioxidative [1, 2],antimalarial [3, 4],antibacterial [5],and antiviral effects [6]. The pyrimidine-based derivatives,e.g. thieno[2,3-d]pyrimidines,were proved to have pharmacological and therapeutic properties in medicinal chemistry,such as anti-inflammatory [7],antibacterial [8, 9],antifungal [10, 11],antitumor [12], antidepressant [13],antiplatelet [14],antihypertensive [15], herbicidal [16] and plant growth regulatory [17] properties.
As phosphorus analogs of α-amino acids and their esters,α-aminophosphonates have received much attention owing to their potential biological activities such as antibacterial,anticancer,as well as insecticidal and herbicidal properties,[18, 19, 20, 21, 22] and were investigated as starting materials for the syntheses of phosphonopeptides. Moreover,if the aminophosphonate subunit was suitably embedded into potential antitumor agents,it could effectively improve their bioactivity [23],e.g. benzothiazole moiety or fluorine moiety containing α-aminophosphonatess [24, 25, 26, 27] were proved to have high antitumor activity.
Based on the concept of bioisosterism and inspired by the above observations,we were led to the proposal to incorporate thieno[2,3-d]pyrimidine ring into the α-aminophosphonates compounds, to construct novel classes of thieno[2,3-d]pyrimidine moiety containing α-aminophosphonates derivatives,and to evaluate their antitumor activity. Therefore,two series of such compounds,incorporating the α-aminophosphonates and the thieno[2,3-d]pyrimidines,were designed and synthesized and their antitumor properties were investigated.
Twenty-two new diethyl or diisopropyl [(3-substituted or 4- substituted or 2,4-disubstituted) (2-methyl-6-ethyl-4-oxo-4-H-thieno[ 2,3-d]pyrimidin-3-yl-amino) methyl] phosphonates were synthesized and their in vitro antitumor activity under cell membrane conditions were evaluated by the MTT method. Compounds 6ma-6mk and 6na-6nk,as well as the intermediates 4a-4k,were tested for their anti-proliferation activity against three human cancer cell lines (EC109,HepG2 and MGC-803) at different concentrations. This represents the first report about the synthesis and in vitro antitumor activity evaluation of thieno[2,3-d]pyrimidines containing α-aminophosphonates derivatives.
2. ExperimentalAll starting materials were commercially available and analytically pure. 1H NMR spectra were recorded on a BRUKER DPX-400 spectrometer (Bruker Company,Germany),using TMS as an internal standard and CDCl3 as a solvent. Chemical shifts (δ values) and coupling constants (J values) are given in pp mand Hz, respectively. TLC analysis was carried out on silica gel plates GF254 (Qindao Haiyang Chemical,China). High-resolution mass spectra were performed on an ESI Q-TOF MS spectrometer (Micromass, England). Melting points were determined on an XT4A apparatus (Beijing Keyi Electro-optic Instrument Plant,China) and were uncorrected.The intensity datawere collectedonanAgilent xcalibur Eos-II- CCD-diffractometer (Agilent Technologies).
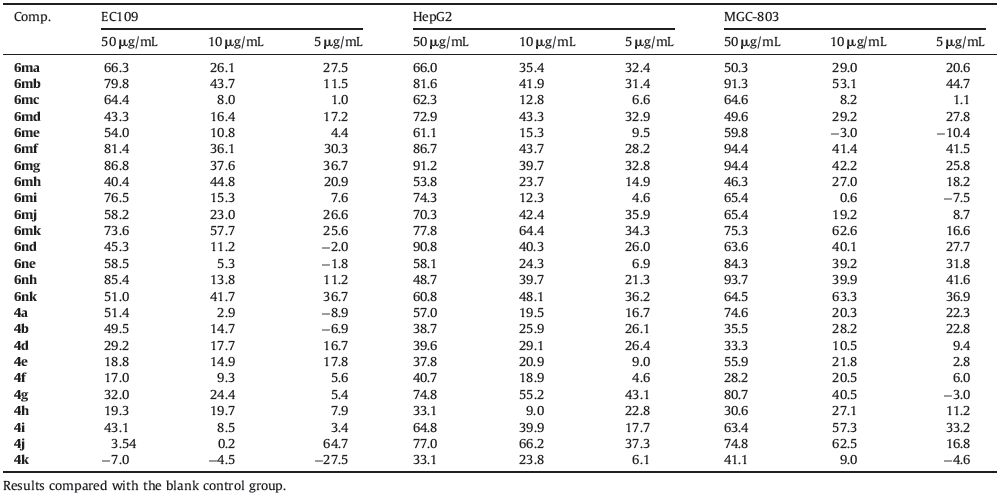
Twenty-two α-aminophosphonates derivatives (6ma-6mk and 6na-6nk) were prepared according to the reaction sequences shown in Scheme 1. The starting materials,ethyl 2-amino-5- ethylthiophene-3-carboxylate (1) [28] and dialkyl phosphite (5), were prepared according to the literature methods. The target analogs were easily obtained in high yields with the established reaction conditions. The acetylation of compound (1) with acetyl chloride in acetonitrile gave the ethyl ester of 2-acetylamino-5- ethylthiophene-3-carboxylic acid (2) [29]. The acetylated intermediate (2) then underwent condensation-cyclization reactions with hydrazine hydrate to afford compound (3),3-amino-6-ethyl- 2-methylthieno[2,3-d]pyrimidin-4-one,in good yield [30]. The Schiff base type key intermediates (4),obtained by the reaction of (3) with various substituted benzaldehydes,reacted with different dialkyl phosphite (5) under nitrogen atmosphere to generate the corresponding α-aminophosphonates derivatives (6ma-6mk and 6na-6nk). All the new compounds were characterized using 1H NMR and 13C NMR,mass spectrometry and high resolution mass spectrometry,respectively (see Supporting information for structure characterization,yields and melting points).
|
Download:
|
| Scheme 1.Synthetic routes for the targeted compounds. | |
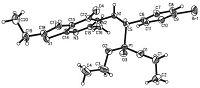
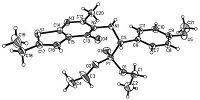
Fortunately,the single crystals of compound 6mi and 6mj were obtained. The molecular structures of the two compounds are illustrated in Figs. 1 and 2. Colorless single crystals of the compound 6mi (0.35 mm × 0.30 mm × 0.30 mm) and 6mj (0.30 mm × 0.25 mm × 0.22 mm) were selected for X-ray diffraction analysis. The data were collected on an Agilent xcalibur Eos-II-CCD-diffractometer equipped with a graphite-monochromatic Cu Kα radiation (λ = 1.5418Å ) by using a ω scan mode in the range of 4.46<θ<72.288 for 6mi and 4.42<θ<67.078 for 6mj at 291(2) K. The entire structure was solved by the direct methods using the SHELXS-97 program [31] and refined by the full-matrix leastsquares method on F2 with anisotropic thermal parameters for all non-hydrogen atoms using SHELXL-97. [32] For compound 6mi,a total of 8957 reflections were recorded and 4476 were unique (Rint. = 0.0225),among which 4245 (-13 ≤ h ≤ 13,-9 ≤ k ≤ 14, -19 ≤ l ≤ 21) were observed. For compound 6mj,a total of 8872 reflections were recorded and 4183 were unique (Rint. = 0.0232),among which 3930 (-12 ≤ h ≤ 8,-14 ≤ k ≤ 13, -19 ≤ l ≤ 21) were observed. X-ray diffraction analysis revealed that the thieno[2,3-d]pyrimidine ring in two compounds did not exhibit good coplanarity and the molecular structure is stabilized by intermolecular N(1)-H(1)···N(3) hydrogen bonds. Thereby,the structure of 6mi and 6mj were further confirmed.
|
Download:
|
| Fig. 1.X-ray structure of compound 6mi. | |
|
Download:
|
| Fig. 2.X-ray structure of compound 6mj. | |
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to investigate in vitro cytotoxic activity. Human esophageal carcinoma cells (EC109),human hepatocarcinoma cells (HepG2) and human gastric carcinoma cells (MGC-803) seeded into the 96-well plate (100 μL each well) were incubated at 37 ℃ under 5% CO2 and 95% O2 until cell adherence was observed. Cells were exposed to suspension of compounds 6ma-6mk,6na-6nk, as well as the intermediates 4a-4k at different concentrations. After 48 h,20 μL of MTT with a concentration of 5 μg/mL was added into the 96-well plate and incubated for 4 h at 37 ℃. Then,the supernatant was aspirated, 150 μL of DMSO was added to each well,and the absorbance was measured at 490 nm. The formula for calculating inhibition of cell proliferation was as follows: inhibition of cell proliferation rate (%) = (OD value of the control group - OD value of drug group)/ control OD value × 100%. This was used to plot an inhibition curve.
3. Results and discussion
The in vitro cytotoxic activity of compounds 6ma-6mk,6na-6nk, as well as the intermediates 4a-4k against human esophageal carcinoma cells (EC109),human hepatocarcinoma cells (HepG2), human gastric carcinoma cells (MGC-803) was evaluated by the MTT method and the potency was expressed as inhibition rate. The results are summarized in Table 1.
|
|
Table 1 Antitumor activity of target compounds (inhibitory ratio/%). |
Results in Table 1 show that the changes of substituents affected the antitumor activity. Among analogs 6ma-6mk and 6na-6nk,even intermediates Schiff bases 4a-4k,compounds 6ma-6mk and 6na-6nk have a relatively higher antitumor activity than that of Schiff bases 4a-4k. These observations indicate that the introduction of phosphoryl group improves antitumor activity to EC109,HepG2 and MGC-803 cells.
The antitumor data also indicate that the substitutes of phosphonate has no apparent influence on antitumor activity to EC109,HepG2 and MGC-803 cells. For example,when R was 4-NO2 and R' was Et,compound 6me exhibited moderate antitumor activity with an inhibition rate of 54.0% against EC109 cells at 50 μg/mL; the antitumor activity of 6ne,where the phosphonate was substituted by an i-Pr,was little higher than the activity of 6me in EC109 with the inhibition rate of 58.5%. Compound 6mk with 2-Cl (R),4-Cl(R),and Et (R') has antitumor activity in HepG2 cells,with an inhibition rate of 77.8% at the concentration of 50 μg/mL. Compound 6nk with 2-Cl (R),4-Cl(R),and i-Pr (R') has a lower antitumor activity (60.8%) than that of 6mk. This same trend was observed for compounds 6md/6nd and 6mh/6nh against EC109,HepG2 and MGC-803 cells.
Compounds 6mg (R:4-Cl,R':Et) and 6nh (R:3-Br,R':i-Pr) showed potent inhibition against EC109 cells with the inhibition rate of 86.8% and 85.4% at 50 μg/mL,respectively. Compounds 6mg (R:4-Cl,R':Et) and 6nd (R:3-NO2,R':i-Pr) showed potent inhibition against HepG2 cells with the inhibition rate of 91.2% and 90.8% at 50 μg/mL,respectively. Compounds 6mb (R:3-CH3,R':Et), 6mf (R:3-Cl,R':Et),6mg (R:4-Cl,R':Et) and 6nh (R:3-Br,R':i-Pr) showed potent inhibition against MGC-803 cells with the inhibition rate of 91.3%,94.4%,94.4% and 93.7% at 50 μg/mL, respectively. It is worth mentioning that compound 6mg presented the highest inhibition against EC109 (IC50 = 10.82 μ'''mol/ L),HepG2 (IC50 = 10.44 μmol/L) and MGC-803 (IC50 = 10.59 μmol/L) cells.
Analogs 6ma-6mk and 6na-6nk,with different substituents on the phenyl ring,showed various degrees of inhibitory activity against EC109,HepG2 and MGC-803 cells. Compounds 6mb/6nb and 6mc/6nc with hydrophobic alkyl chains (-CH3) exerted moderate inhibitory activity except for 6mb. Analogs 6md/6nd and 6me/6ne with strong electron-withdrawing groups -NO2 as meta-substitutions or para-substitutions showed favorable antibacterial activity,but only 6nd demonstrated potent inhibition against HepG2 cells. Analogs 6mf/6nf (3-Cl),6mg/6ng (4-Cl),6mh/6nh (3-Br) and 6mi/6ni (4-Br) showed different inhibitory activity against EC109,HepG2 and MGC-803 cells. The antitumor inhibition of 6mf/6nf (3-Cl) and 6mg/6ng (4-Cl) with the substituents at meta-or para-position were better than 6mh/6nh (3-Br) and 6mi/6ni (4-Br),respectively. No activity improvementwas exhibited by 6mj/6nj (4-OCH3) and 6mk/6nk (2,4-dichloride) compared to 6ma(H). The results indicate that different substituents at different positions of the benzene ring significantly affect the antitumor activity of these compounds and the introduction of the electron-withdrawing groups to the benzene ring led to an increase of the antitumor activity. As to compound 6mg,it is possible that the electronic and steric effects of chlorine atoms were more complementary to the receptors. Further studies will focus on structural optimization and structure-activity relationships of these compounds.
4. ConclusionIn summary,two series of novel α-aminophosphonates derivatives containing thieno[2, 3-d]pyrimidines were synthesized and their antitumor activity in vitro against EC109,HepG2 and MGC- 803 cells has been evaluated. Biological assays revealed that the substitutions on the phenyl ring influenced the antitumor activity remarkably,while the substitutes of phosphonate had no apparent influence on the antitumor activity. Analogs 6mf/6nf (3-Cl) and 6mg/6ng (4-Cl) with the substituents at meta- or para-position showed better inhibition against all of the tested tumor cells. Amongst them,compound 6mg demonstrated excellent inhibitory effect against HepG2 (91.2%) and MGC-803(94.4%) cells. These results are useful for the design of more potent novel antitumor compounds and further study are ongoing.
AcknowledgmentThis work was supported by the National Natural Sciences Foundations of China (Nos. 21171149,21105091). We are grateful to Pro. Yanbing Zhang’ group for performing in vitro antitumor activity evaluation.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.026.
| [1] | Y. Kotaiah, N. Harikrishna, K. Nagaraju, C. Venkata Rao, Synthesis and antioxidant activity of 1,3,4-oxadiazole tagged thieno[2,3-d] pyrimidine derivatives, Eur. J. Med. Chem. 58 (2012) 340-345. |
| [2] | H.A. Stefani, C.B. Oliveira, R.B. Almeida, et al., Dihydropyrimidin-(2H)-ones obtained by ultrasound irradiation: a new class of potential antioxidant agents, Eur. J. Med. Chem. 41 (2006) 513-518. |
| [3] | H. Kikuchi, K. Yamamoto, S.Horoiwa, et al., Exploration of a newtype of antimalarial compounds based on febrifugine, J. Med. Chem. 49 (2006) 4698-4706. |
| [4] | B. Singh, A. Maheshwari, G. Dak, K. Sharma, G.L. Talesara, Studies of antimicrobial activities of some 4-thiazolidinone fused pyrimidines, [1,5]-benzodiazepines and their oxygen substituted hydroxylamine derivatives, Indian J. Pharm. Sci. 72 (2010) 607-612. |
| [5] | S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian, M. Davoodnejad, A novel and efficient synthesis of pyrazolo[3,4-d]pyrimidine derivatives and the study of their anti-bacterial activity, Chin. Chem. Lett. 24 (2013) 629-632. |
| [6] | H.N. Hafez, H.A. Hussein, A.R. El-Gazzar, Synthesis of substituted thieno[2,3-d]pyrimidine-2,4-dithiones and their S-glycoside analogues as potential antiviral and antibacterial agents, Eur. J. Med. Chem. 45 (2010) 4026-4034. |
| [7] | V. Alagarsamy, S. Meena, K.V. Ramseshu, et al., Synthesis, analgesic, anti-inflammatory, ulcerogenic index and antibacterial activities of novel 2-methylthio-3-substituted-5,6,7,8-tetrahydrobenzo (b) thieno[2,3-d]pyrimidin-4(3H)-ones, Eur. J. Med. Chem. 41 (2006) 1293-1300. |
| [8] | M.D. Bhuiyan, K.M. Rahman, M.D. Hossain, et al., Synthesis and antimicrobial evaluation of some new thienopyrimidine derivatives, Acta Pharm. 56 (2006) 441-450. |
| [9] | M.R. Prasad, J. Prashanth, K. Shilpa, D.P. Kishore, Synthesis and antibacterial activity of some novel triazolothieno-pyrimidines, Chem. Pharm. Bull. (Tokyo) 55 (2007) 557-560. |
| [10] | H.M. Aly, N.M. Saleh, H.A. Elhady, Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents, Eur. J. Med. Chem. 46 (2011) 4566-4572. |
| [11] | B.V. Ashalatha, B. Narayana, K.K. Vijaya Raj, N.S. Kumari, Synthesis of some new bioactive 3-amino-2-mercapto-5,6,7,8-tetrahydro[1]benzothieno[2,3-d]pyrimidin-4(3H)-one derivatives, Eur. J. Med. Chem. 42 (2007) 719-728. |
| [12] | X.J. Song, P. Yang, H. Gao, et al., Facile synthesis and antitumor activity of novel 2-trifluoromethyl-thieno[2,3-d]pyrimidine derivatives, Chin. Chem. Lett. 25 (2014) 1006-1010. |
| [13] | T. George, C.L. Kaul, R.S. Grewal, R. Tahilramani, Antihypertensive and monoamine oxidase inhibitory activity of some derivatives of 3-formyl-4-oxo-4Hpyrido[ 1,2-a]pyrimidine, J. Med. Chem. 14 (1971) 913-915. |
| [14] | O. Bruno, C. Brullo, A. Ranise, et al., Synthesis and pharmacological evaluation of 2,5-cycloamino-5H-[1]benzopyrano[4,3-d]pyrimidines endowed with in vitro antiplatelet activity, Bioorg. Med. Chem. Lett. 11 (2001) 1397-1400. |
| [15] | R.K. Russell, J.B. Press, R.A. Rampulla, et al., Thiophene systems. 9. Thienopyrimidinedione derivatives as potential antihypertensive agents, J. Med. Chem. 31 (1988) 1786-1793. |
| [16] | H. Liu, H.Q. Wang, Z.J. Liu, Synthesis and herbicidal activity of novel pyrazolo[3,4-d]pyrimidin-4-one derivatives containing aryloxyphenoxypropionate moieties, Bioorg. Med. Chem. Lett. 17 (2007) 2203-2209. |
| [17] | J.M. Wang, T. Asami, S. Yoshida, N. Murofushi, Biological evaluation of 5-substituted pyrimidine derivatives as inhibitors of brassinosteroid biosynthesis, Biosci. Biotechnol. Biochem. 65 (2001) 817-822. |
| [18] | W.M. Abdou, R.F. Barghash, M.S. Bekheit, Carbodiimides in the synthesis of enamino-and α-aminophosphonate as peptidomimetics of analgesic/antiinflammatory and anticancer agents, J. Arch. Pharm. Chem. Life Sci. 345 (2012) 884-895. |
| [19] | U.R.S. Arigala, C. Matcha, K.R. Yoon, Zn(OAc)2·2H2O-catalyzed synthesis of α-aminophosphonate under neat reaction, Heteroat. Chem. 23 (2012) 160-165. |
| [20] | M.V.N. Reddy, A. Balakrishna, M.A. Kumar, et al., One-Step synthesis and bioassay of N-phosphoramidophosphonates, Chem. Pharm. Bull. 57 (2009) 1391-1395. |
| [21] | X.P. Rao, Z.Q. Song, L. He, Synthesis and antitumor activity of novel α-aminophosphonate from diterpenic dehydroabietylamine, Heteroat. Chem. 19 (2008) 512-516. |
| [22] | S.R. Devineni, S. Doddaga, R. Donka, N.R. Chamarthi, CeCl3·7H2O-SiO2: catalyst promoted microwave assisted neat synthesis, antifungal and antioxidant activities of a-diaminophosphonates, Chin. Chem. Lett. 24 (2013) 759-763. |
| [23] | M. Gnant, P. Clézardin, Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature, Cancer Treat. Rev. 38 (2012) 407-415. |
| [24] | B.A. Song, G.P. Zhang, S. Yang, D.Y. Hu, L.H. Jin, Synthesis of N-(4-bromo-2-trifluoromethylphenyl)-1-(2-fluorophenyl)-O,O-dialkyl-α-aminophosphonate under ultrasonic irradiation, Ultrason. Sonochem. 13 (2006) 139-142. |
| [25] | L.H. Jin, B.A. Song, G.P. Zhang, et al., Synthesis, X-ray crystallographic analysis, and antitumor activity of N-(benzothiazole-2-yl)-1-(fluorophenyl)-O,O-dialkyl-aaminophosphonates, Bioorg. Med. Chem. Lett. 16 (2006) 1537-1543. |
| [26] | B. Kaboudin, N.J. As-habei, Microwave-assisted synthesis of a-aminophosphinic acids from hypophosphorus acid salts under solvent free conditions, Tetrahedron Lett. 44 (2003) 4243-4245. |
| [27] | B.C. Ranu, A. Hajra, A simple and green procedure for the synthesis of a-aminophosphonate by a one-pot three-componentcondensation of carbonyl compound, amine and diethyl phosphite without solvent and catalyst, Green Chem. 4 (2002) 551-554. |
| [28] | S. Liu, Studies on Synthesis and Properties of Thienopyrimidine and Pyridopyrimidine Derivatives, Master thesis, Jiangxi Normal University, Nanchang, 2009. |
| [29] | K. Clausen, S.O. Lawesson, Studies on organophosphorus compounds synthesis of ethyl-2-(thioacylamino)-5-ethyl-3-thiophenecarboxylates and 2-substituted 6-ethylthieno [2,3-d] thiazine-4-thiones, Nouv. J. Chem. 4 (1980) 43-46. |
| [30] | R.V. Chambhare, B.G. Khadse, A.S. Bobde, et al., Synthesis and preliminary evaluation of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d] pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d] pyrimidin-4-ones as antimicrobial agents, Eur. J. Med. Chem. 38 (2003) 89-100. |
| [31] | G.M. Sheldrick, SHELXS-97, Program for the Solution of Crystal Structure, University of Göttingen, Germany, 1997. |
| [32] | G.M. Sheldrick, SHELXL-97, Program for the Crystal Structure Solution and Refinement, University of Göttingen, Germany, 1997. |