The Strecker reaction [1] provides one of the most efficient methods for the synthesis of α-aminonitriles,which are useful intermediates in the preparation of many amino acids and various nitrogen containing heterocycles such as imidazoles,thiadiazoles, etc. [2] and other biologically useful molecules such as saframycin- A,a natural product with antitumor activity,or phthalascidin,a synthetic analog,which exhibits even greater potency [3]. They are usually prepared by the nucleophilic addition of a cyanide anion to imine intermediates. Numerous methods have been reported describing the preparation of α-aminonitriles [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. The classical Strecker reaction is generally carried out with hydrogen cyanide or alkaline cyanides in aqueous solution. To overcome these limitations,several modifications of the Strecker reaction have been reported [16]. These modifications use alternative cyanating sources,such as hydrogen cyanide (HCN) [17],potassium cyanide (KCN) [18],sodium cyanide (NaCN),trimethylsilyl cyanide (TMSCN) [19],diethylphosphorocyanidate ((OEt)2P(O)CN) [20], bis(dialkyl amino cyano)borans,diethyl aluminum cyanide (Et2AlCN) [21] and tributyltin cyanide (Bu3SnCN),etc. [22] under various reaction conditions. Among these cyanide agents, trimethylsilyl cyanide,has proven to be relatively safe,easy to handle,highly soluble in organic solvents,and more effective as a cyanide anion source for the nucleophilic addition of imines under mild conditions compared to other cyanating reagents [23]. Other modifications to the Strecker reaction use catalysts,such as InCl3 [24],BiCl3 [25],montmorillonite KSF clay [26],silica bonded scandium (III) [27],SO4/ZrO2 [28],ferric perchlorate [29], Fe(Cp)2PF6 [30],InI3 [31],I2 [32],K5CoW12O40·3H2O [33],vanadyl triflate [34],Fe3O4 [35],guanidine hydrochloride [36],xanthan sulfuric acid [37], [bmim]BF4 [38],silica sulfuric acid [39], hydrophobic sulfonic acid based nanoreactors [40] and silicabonded S-sulfonic acid [41] under various conditions. However, many of these protocols suffer from disadvantages such as strongly acidic conditions,unsatisfactory yields,and longer reaction times in addition to tedious aqueous workups leading to the generation of a large amount of toxic waste and use of stoichiometric or relatively expensive reagents. Therefore,there is motivation to explore a milder,safer,and more efficient catalyst for the synthesis of α-aminonitriles within short reaction times. T3P® is a well known green coupling reagent and a powerful water scavenger with several advantages,including low toxicity,low allergic potential,broad functional group tolerance,generation of products in excellent yield,purity,and easy workup procedures due to the formation of water soluble byproducts [42, 43, 44]. Furthermore T3P® has been investigated extensively in the preparation of various heterocyclic compounds.
Though several works focused on the synthesis of α-aminonitriles have been reported,the preparation of α-aminonitriles from the reaction of heterocyclic aldehydes has not been extensively explored. Owing to their synthetic and biological value,there is a need to find new,accessible,cheaper,and more efficient approaches to the synthesis of α-aminonitriles. Heterocyclic skeletons serve as ideal scaffolds on which pharmacophores can be appended to yield potent and selective drugs. In view of this research and our desire to develop α-aminonitrile structures,we have designed and synthesized a series of α-aminonitriles with different heterocyclic and aromatic aldehydes. Herein,we report for the first time the finding of our investigations for the synthesis of α-aminonitriles (4a-t) by one-pot three-component coupling of various heterocyclic aldehydes/aromatic aldehydes,secondary amines,and trimethylsilyl cyanide catalyzed by commercially available,inexpensive propylphosphonic anhydride (T3P®).
2. ExperimentalAll reactions were performed under nitrogen conditions in anhydrous solvents such as acetonitrile,dichloromethane,and tetrahydrofuran. All reactions were monitored by TLC analysis using Merck silica gel 60 F254 plates with fluorescent indicator (254 nm) and visualized with a UV lamp. All commercially available reagents such as 5-fluoro-3-methyl-1H-indole-2-carbaldehyde, 4-(4-morpholinyl) benzaldehyde,6-bromopicolinaldehyde, thiophene-2-carbaldehyde,5-bromo thiophene-2- carbaldehyde,and propylphosphonic anhydride (≥50 wt% in ethyl acetate) were procured from Aldrich,Across Organics,and Apollo Scientific companies and used as received without further purification.
Melting points were recorded on Mel-Temp apparatus and are uncorrected. All the infrared spectra of the title compounds were recorded on a Bruker Alpha-Eco ATR-FTIR (Attenuated total reflection-Fourier transform infrared) interferometer with a single reflection sampling module equipped with ZnSe crystal. 1H NMR and 13C NMR were recorded on Bruker DRX 400 and 500 MHz spectrometers using TMS as internal standard. Chemical shifts (δ) are reported in ppm. Coupling constants are reported in Hz. Mass spectra were recorded on a Finnigan MAT 1020 mass spectrometer operating at 70 eV.
2.1. General procedureTo a stirred solution of 5-fluoro-3-methyl-1H-indole-2-carbaldehyde (177 mg,1 mmol) and morpholine (96 mg,1.1 mmol) in acetonitrile (7 mL) was added trimethylsilyl cyanide (119 mg, 1.2 mmol) followed by T3P (63 mg,20 mol%) at ambient temperature. The reaction mixture was stirred for an approximate time and monitored by TLC. After completion of the reaction,the reaction mixture was diluted with water (10 mL) and extracted with ethyl acetate (3× 15 mL). The organic layers were combined,washed with brine,dried over anhydrous sodium sulfate,and concentrated under reduced pressure. The resulting residue was purified by using 100-200 mesh silica and eluting with 13%-15% ethyl acetate in n-hexane to afford pure 2-(5-fluoro-3-methyl-1H-indol-2-yl)-2- morpholinoacetonitrile as a solid (4a) in 95% (263 mg) yield. This procedure is applied to the other reactions (4b-t).
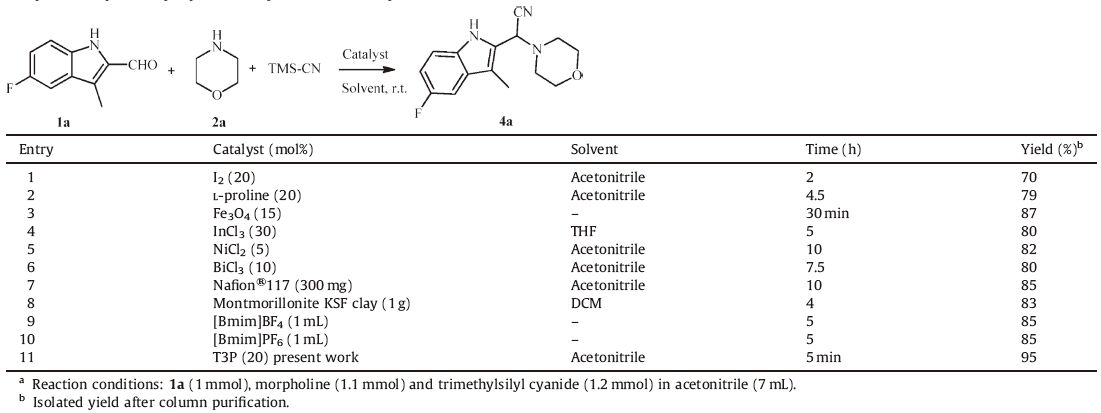
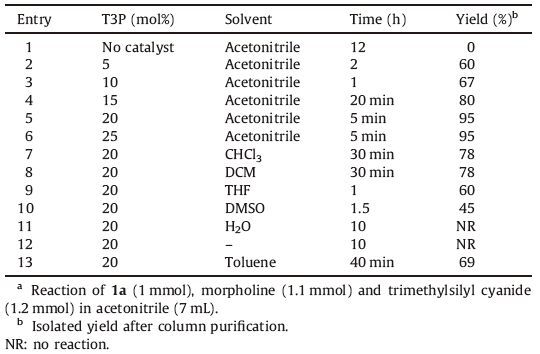
3. Results and discussionTo find the best reaction conditions,we first examined the effect of the catalyst for the Strecker three component reaction of 5- fluoro-3-methyl-1H-indole-2-carbaldehyde (1a),morpholine (2a) and trimethylsilyl cyanide in presence of T3P® (20 mol%) at an ambient temperature as a model reaction,and the corresponding 2-(5-fluoro-3-methyl-1H-indol-2-yl)-2-morpholinoacetonitrile (4a) was obtained in 95% yield (Table 1). This is because of rapid formation and activation of imine intermediate with T3P® and formation of water soluble by-products. A set of experiments were carried out by changing the amount of catalyst (Table 2). The use of 20 mol% (Table 2,entry 5) T3P® is sufficient to promote the reaction and no additives are required for complete conversion of starting materials to the desired product. It is remarkable to note that no improvements were observed in the case of reaction rate and yields by increasing the amount of catalyst from 20 mol% to 25 mol% (Table 2,entries 5 and 6). The use of lower amount of catalyst (down to 5 mol%) (Table 2,entry 2) also worked when prolonged reaction time to 2 h. In the absence of T3P® (Table 2, entry 1) even after 12 h no reaction was observed. We also screened the effects of various solvents such as CHCl3,DCM,THF, DMSO,H2O,toluene,CH3CN and without solvent (Table 2). Among these solvents,acetonitrile was found to be the best catalyst.
|
|
Table 1 Comparison of previously reported catalysts with our catalyst.a |
|
|
Table 2 Effect of the catalyst and solvent on the synthesis of α-aminonitriles.a |
At the optimized conditions,we extended the scope of the T3P1 catalyzed synthesis of a-animonitriles (4a-t) with different heterocyclic aldehydes (1a-f)/aromatic aldehydes (1g-t),secondary amines (2a-t) and TMS-CN (Table 3).
|
|
Table 3 T3P® catalyzed synthesis of α-aminonitriles with trimethylsilyl cyanide. |
In general,the reactions are very fast and no undesired products were formed,such as cyanohydrin trimethylsilyl ether (adduct between aldehyde and trimethylsilyl cyanide). We checked the reactivity of the catalyst with cyanating reagents such as sodium cyanide,potassium cyanide,and tributyltin cyanide in the same reaction conditions. In all cases we obtained corresponding α-aminonitriles in high to excellent yields with only slightly longer reaction time. In the same way,a wide range of structurally varied aldehydes were coupled with 2-amines (e.g. morpholine, pyrrolidine and piperdine) and trimethylsilyl cyanide in a one pot operation using this procedure to obtain α-aminonitriles in 86%-95% yields. These three component coupling reactions were carried out at an ambient temperature with high selectivity. We found that aldehydes in general,including heterocyclic and aromatic units,participate rapidly in this reaction,whereas acetophenone and benzophenone largely remained inert; longer reaction times and greater amount of catalyst also failed to make significant improvements. This method is equally effective with aromatic aldehydes bearing an electron withdrawing or electron releasing substituent in the aromatic ring. The results shown in Table 3 clearly indicate the scope and generality of the reaction with respect to the various aldehydes and 2-amines.
4. ConclusionIn conclusion we have developed a new simple,convenient and practicalmethod for the synthesis of α-aminonitriles through a onepot three component coupling reaction of aldehyde,amine and trimethylsilyl cyanide in the presence of catalytic amount of T3P® in acetonitrile at ambient temperature. Here,T3P® is a novel and very effective catalyst. The main advantages of this method are operational simplicity,very short reaction time,low toxicity,and excellent yields of products. This methodology is applicable for a wide range of hetero aromatic aldehydes such as 5-methoxybenzofuran- 2-carbaldehyde,5-fluoro-3-methyl-1H-indole-2-carbaldehyde, 4-(4-morpholinyl)benzaldehyde,6-bromopicolin-aldehyde, thiophene-2-carbaldehyde,and 5-bromothiophene-2-carbaldehyde and various aromatic aldehydes. This procedure will prove to be a viable alternative to the existing ones.
AcknowledgmentsThe authors are thankful to the Board of Research in Nuclear Sciences (BRNS),Mumbai,India for providing the financial support (No. 2012/37C/33/BRNS). We also grateful to the University Grant Commission (UGC),New Delhi for the award of JRF fellowship to S.S. Reddy and B.R.P. Reddy. Finally authors are also thankful to Dr. R.V. Jayanth Kasyap,Department of English,Yogi Vemana University,Kadapa,A.P.,India for verifying the grammatical and linguistic correctness.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.021.
| [1] | Y.M. Shafran, V.A. Bakulev, V.S. Mokrushin, Synthesis and properties of α-aminonitriles, Russ. Chem. Rev. 58 (1989) 148–162. |
| [2] | D.A. Martier, W.L. Owens, D. Comer, et al., Antihypertensive agents. Synthesis and biological properties of 2-amino-4-aryl-2-imidazolines, J. Med. Chem. 16 (1973) 901–908. |
| [3] | R.O. Duthaler, Recent developments in the stereoselective synthesis of α-aminonitriles, Tetrahedron 50 (1994) 1539–1650. |
| [4] | N. Baskar, C. Fortenberry, A.R. Bunce, Synthesis of aminonitriles under mild catalytic, metal-free conditions, Tetrahedron Lett. 55 (2014) 379-381. |
| [5] | A. Sengupta, C. Su, C. Bao, C.T. Tai, K.P. Loh, Graphene oxide and its functionalized derivatives as carbocatalysts in the multicomponent Strecker reaction ketones, ChemCatChem 6 (2014) 2507-2511. |
| [6] | Y. Wen, Y. Xiong, L. Chang, et al., Chiral bisformamides as effective organocatalysts for the asymmetric one pot three component Strecker reaction, J. Org. Chem. 72 (2007) 7715-7719. |
| [7] | J. Huang, X. Liu, Y. Wen, B. Qin, X. Feng, Enantioselective Strecker reaction of phosphinoyl ketoimines catalyzed by in situ prepared chiral N,N'-dioxides, J. Org. Chem. 72 (2007) 204-208. |
| [8] | S.C. Pan, B. List, Catalytic asymmetric three component acyl-Strecker reaction, Org. Lett. 9 (2007) 1149-1151. |
| [9] | M. Negru, D. Schollmeyer, H. Kunz, Enantioselective Strecker reaction catalyzed by an organocatalyst lacking a hydrogen-bond donar function, Angew. Chem. Int. Ed. Engl. 46 (2007) 9339-9341. |
| [10] | G.K.S. Prakash, T. Mathew, C. Panja, et al., Gallium (III) triflate catalyzed efficient Strecker reaction of ketones and their fluorinated analogs, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 3703-3706. |
| [11] | M.Z. Kassaee, H. Masrouri, F. Movahedi, Sulfamic acid functionalized magnetic Fe3O4 nanoparticles as an efficient and reusable catalyst for one pot synthesis of α-aminonitriles in water, Appl. Catal. A: Gen. 395 (2011) 28-33. |
| [12] | M.N. Sefat, D. Saberi, K. Niknam, Preparation of silica-based ionic liquid an efficient and recyclable catalyst for one pot synthesis of α-aminonitriles, Catal. Lett. 141 (2011) 1713-1720. |
| [13] | T. Rahi, M. Bagherrnejad, K. Niknam, Synthesis of α-aminonitriles using silica bonded N-propylpiperazine sulfamic acid as a recyclable catalyst, Chin. Chem. Lett. 23 (2012) 1103-1106. |
| [14] | A.R. Hajipour, I.M. Dehbane, An efficient one pot synthesis of α-aminonitriles using ecofriendly Lewis acidic ionic liquid cholin chloride 2ZnCl2, Iran. J. Catal. 2 (2012) 147-151. |
| [15] | S. Ghasemi, M. Bagherrnejad, K. Niknam, Sulfuric acid {[3-(3silicapropyl)sulfanyl] propyl} ester as a recyclable solid acid catalyst for the synthesis of a-amino nitries, Iran. J. Catal. 3 (2013) 165-169. |
| [16] | R. Warmuth, T.E. Munsch, R.A. Stalker, B. Li, A. Beatty, Enantioselective synthesis of benzocyclic a, a-dialkyl-amino acids: new insight into the solvent dependent stereoselectivity of the TMSCN addition to phenylglycinol derived imines, Tetrahedron 57 (2001) 6383-6397. |
| [17] | D. Enders, J.P. Shilvock, Some recent applications of a-aminonitrile chemistry, Chem. Soc. Rev. 29 (2000) 359-373. |
| [18] | S. Kobayashi, H. Ishitani, Catalytic enantioselective addition to imines, Chem. Rev. 99 (1999) 1069-1094. |
| [19] | B.A. Bhanu Prasad, A. Bisal, V.K. Singh, Trimethylsilyl cyanide addition to aldimines and its application in the synthesis of (S)-phenylglycine methyl ester, Tetrahedron Lett. 45 (2004) 9565-9567. |
| [20] | S. Harusawa, Y. Hmada, T. Shiori, Diethyl phosphorocyanidated (DEPC). A novel reagent for the classical Strecker's a-aminonitrile synthesis, Tetrahedron Lett. 20 (1979) 4663-4666. |
| [21] | S. Nakamura, N. Sato, M. Sugimoto, T. Toru, A new approach to enantioselective cyanation of imines with Et3AlCN, Tetrahedron Asymmetry 15 (2004) 1513-1516. |
| [22] | P. Vachal, E.N. Jacobsen, Structure-based analysis and optimization of a highly enantioselective catalyst for the Strecker reaction, J. Am. Soc. 124 (2002) 10012-10014. |
| [23] | A. Baeza, C. Najera, J.M. Sansano, Solvent-free synthesis of racemic α-aminonitriles, Synthesis (2007) 1230-1234. |
| [24] | B.C. Ranu, S.S. Dey, A. Hajra, Indim trichloride catalyzed one-step synthesis of aaminonitriles by a three-component condensation of carbonyl compounds, amines and potassium cyanide, Tetrahedron 58 (2002) 2529-2532. |
| [25] | S.K. De, R.A. Gibbs, Bismuth trichloride catalyzed synthesis of α-aminonitriles, Tetrahedron Lett. 45 (2004) 7407-7408. |
| [26] | J.S. Yadav, B.V.S. Reddy, B. Eeshwaraiah, M. Srinivas, Montmorillonite KSF clay catalyzed one-pot synthesis of α-aminonitriles, Tetrahedron 60 (2004) 1767-1771. |
| [27] | B. Karimi, A.A. Safari, One-pot synthesis of α-aminonitriles using a highly efficient and recyclable silica-based scandium (III) interphase catalyst, J. Organomet. Chem. 693 (2008) 2967-2970. |
| [28] | B.M. Reddy, B. Thirupathi, M.K.K. Patil, Highly efficient promoted zirconia solid acid catalysts of α-aminonitriles using trimethylsilyl cyanide, J. Mol. Catal. A: Chem. 307 (2009) 154-159. |
| [29] | H.A. Oskooie, M.M. Heravi, A. Sadnia, F. Jannati, F.K. Behbahabi, Ferric perchloratecatalyzed one-pot synthesis of α-aminonitriles using trimethylsilylcyanide, Synth. Commun. 37 (2007) 2543-2548. |
| [30] | N.H. Khan, S. Agrawal, R.I. Kureshy, et al., Fe(Cp)2PF6 catalyzed efficient Strecker reactions of ketones and aldehydes under solvent-free conditions, Tetrahedron Lett. 49 (2008) 640-644. |
| [31] | Z.L. Shen, S.J. Ji, T.P. Loh, Indium(III) iodide-mediated Strecker reaction in water: an efficient and environmentally friendly approach for the synthesis of α-aminonitriles via a three-component condensation, Tetrahedron 64 (2008) 8159-8163. |
| [32] | S.H. Wang, L.F. zhao, Z.M. Du, iodide catalyzed three-component Strecker-type synthesis of α-aminonitriles from aldehydes, amines and tributyltin cyanide, Chin. J. Chem. 24 (2006) 135-137. |
| [33] | E. Rafiee, A. Azad, K5CoW12O40·3H2O: heterogeneous catalyst for the Streckertype aminative cyanation of aldehydes and ketones, Synth. Commun. 37 (2007) 1127-1132. |
| [34] | S.K. De, Vanadyl triflate as an efficient and recyclable catalyst for the synthesis of α-aminonitriles, Synth. Commun. 35 (2005) 1577-1582. |
| [35] | M.M. Mojtahedi, S. Abaee, T. Alishiri, Superparamagnetic iron oxide as an efficient catalyst for the one-pot, solvent-free synthesis of α-aminonitriles, Tetrahedron Lett. 50 (2009) 2322-2325. |
| [36] | A. Heyderi, A. Arefi, S. Khaksar, R.K. Shiroodi, Guanidine hydrochloride: an active and simple catalyst for Strecker type reaction, J. Mol. Catal. A: Chem. 271 (2007) 142-144. |
| [37] | A. Shaabani, A. Maleki, M.R. Soudi, H. Mofakham, Xanthan sulfuric acid: a new and efficient bio-supported solid acid catalyst for the synthesis of α-aminonitriles by condensation of carbonyl compounds, amines and trimethylsilylcyanide, Catal. Commun. 10 (2009) 945-949. |
| [38] | J.S. Yadav, B.V.S. Reddy, B. Eeshwaraiah, M. Srinivas, P. Vishnumuythy, Threecomponent coupling reactions in: a facile synthesis of α-aminonitriles, New J. Chem. 27 (2003) 462-465. |
| [39] | W.Y. Chen, J. Lu, Silica sulfuric acid catalyzed one-pot synthesis of α-aminonitriles, Synlett (2005) 2293-2296. |
| [40] | B. Karimi, D. Zareyee, Solvent-free three component Strecker reaction of ketones using highly recyclable and hydrophobic sulfonic acid based nanoreactors, J. Mater. Chem. 19 (2009) 8665-8670. |
| [41] | K. Niknam, D. Saberi, M. Nouri Sefat, Silica-bonded S-sulfonic acid: an efficient and recyclable solid acid catalyst for the three-component synthesis of α-aminonitriles, Tetrahedron Lett. 51 (2010) 2959-2962. |
| [42] | M. Desroses, M. Scobie, T. Helleday, A new concise synthesis of 2,3-dihydroquinazolin-4 (1H)-one derivatives, ChemInform 37 (2013) 3595-3597. |
| [43] | T.H. Ngo, H. Berndt, D. Lentz, H.U. Reissig, Linear and cyclic amides with a thiophene backbone: ultrasound-promoted synthesis and crystal structures, J. Org. Chem. 77 (2012) 9676-9683. |
| [44] | M. Milen, P.A. Balogh, A. Dancso, et al., T3P®-promoted Kabachnik-Fields reaction: an efficient synthesis of a-aminophosphates, ChemCatChem 54 (2013) 5430-5433. |