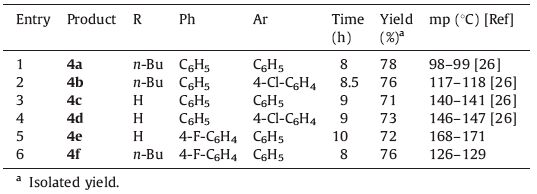
Aminoesters and amino acids are valuable synthetic scaffolds for both medicinal and synthetic organic chemists. A bioreducible linear poly(β-amino ester) as a safe and effective siRNA delivery vehicle have been designed to condense siRNA into nanoparticles and efficiently release it upon entering the cytoplasm [1]. A library of end-modified poly(β-amino ester)s have been reported as gene delivery vehicles. These polymers will provide promising candidates for the systemic treatment of cancer [2]. A series of the β-aminoester class of factor Xa (fXa) inhibitors has been expanded with excellent in vivo anticoagulant activity [3]. Therefore,looking for efficient and concise methods for the synthesis of β-amino ester has commanded vast attention. Synthesis of bioactive compounds should be facile,rapid,highly flexible,and practical in organic synthesis. Multicomponent reactions have been successfully employed to generate molecular complexity starting from simple substrates. Hence,synthetic organic chemists have increasingly focused on the use of MCRs to synthesize a broad range of products [4, 5, 6, 7, 8, 9, 10, 11, 12]. The possibility of accomplishing multicomponent reactions under mild conditions with a heterogeneous catalyst could improve their significant advantages from cost-effectiveness and ecological points of view. The improvement of a catalytic system utilizing inexpensive and commercially available catalysts has been a challenge in organic syntheses. Nanoparticles have undergone extensive examination in the past years. Recently, nanoparticle catalysts have emerged as an alternative method for the development of many considerable organic reactions. Nanocatalysts decrease reaction times and they can be easily recovered from the reaction mixture by standard techniques (filtration, centrifugation) and recycle without losing their activity [13, 14, 15, 16, 17, 18, 19, 20]. In comparison with conventional supports like solid-phase,nanoparticular matrixes have a higher catalyst loading capacity owing to their very large surface area. Among various solid supports,nano silica gel is one of the extensively used surface material supports for different chemical transformations in organic chemistry [21, 22]. Recently,silica-supported ferric chloride has been used as an efficient heterogeneous catalyst for the synthesis 1,4- dihydropyridines [23],xanthenes [24],1,2-dihydro-1-arylnaphtho[ 1,2-e][1, 3]oxazine-3-ones [25]. Recently,the synthesis of cyclic β-aminoesters via the three-component coupling of primary amines,β-ketoesters,and chalcones has been reported using MCRs in the presence of cerium(IV) ammonium nitrate (CAN) as catalyst [26]. Herein,we report that FeCl3/SiO2 nanoparticles acts as an efficient heterogeneous catalyst for the direct synthesis of 4,6-disubstituted 2-alkylaminocyclohexene-1-carboxylic esters by the three-component reaction between primary amines,ethyl acetoacetate and chalcones in ethanol as solvent (Scheme 1).
|
Download:
|
| Scheme 1.Synthesis of 2-aminocyclohex-1-ene-1-carboxylic esters from of primary amines, ethyl acetoacetate and chalcones using FeCl3/SiO2 NPs. | |
All reagents were purchased from Merck and Aldrich and used without further purification. Melting points were measured on an Electrothermal 9200 apparatus. The IR spectra were recorded on an FT-IR Magna 550 apparatus using KBr discs. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance DRX-400 instrument using TMS as the internal standard and CDCl3 as solvent. The elemental analyses (C,H,N) were obtained from a Carlo ERBA Model EA 1108 analyzer. Microscopic morphology of products was examined by SEM (LEO 1455VP).
2.2. Preparation of nano silica-supported ferric chlorideNano silica gel (25 g) and FeCl3·6H2O (2 g) (8% of the weight of nano-SiO2) were vigorously stirred under solvent-free conditions at room temperature for 24 h to achieve a homogeneous adsorption. A yellowish powder was obtained. This powder was heated for 1 h at 100 ℃ to give a brownish powder (‘active’ FeCl3- SiO2 reagent).
2.3. General procedure for the preparation of 2-aminocyclohex-1- ene-1-carboxylic esters (4a-f)A solution of amine (3.9 mmol) and ethyl acetoacetate (3 mmol) in ethanol (4 mL) and FeCl3/SiO2 nanoparticles (0.6 mol%) as catalyst was stirred for 15 min at room temperature. Chalcone (3.3 mmol) was then added to the stirred solution and the stirring was continued for the time periods specified. After completion of the reaction,as indicated by TLC,the mixture was dissolved in CH2Cl2 (20 mL),was filtered and the heterogeneous catalyst was recovered,washed with water,brine,dried (anhydrous Na2SO4),and the solvent was evaporated under reduced pressure. Pure products were obtained by column chromatography on neutral alumina,eluting with a n-hexane-ethyl acetate mixture (90:10,v/v). The structures of the products were fully established on the basis of their 1H NMR,13C NMR and FT-IR spectra.
Ethyl 2-(butylamino)-4-hydroxy-4,6-diphenylcyclohex-1-enecarboxylate (4a): mp 97-100 ℃. IR (KBr,cm-1): υmax 3445.6, 3269.5,3019.7,1628.5,1594.5,1449.0,1H NMR (CDCl3,400 MHz): δ 0.88 (t,3H,J = 6.9 Hz),1.06 (t,3H,J = 7.2 Hz),1.09 (m,2H),1.27 (m,2H),2.28 (dd,1H,J = 14.0,12.0 Hz),2.39 (dd,1H,J = 14.0, 6.9 Hz),2.49 (m,1H),2.80 (m,1H),3.13 (dd,1H,J = 12,6.9 Hz),3.61 (m,2H),4.08 (q,2H,OCH2),6.59 (bs,1H,NH),7.50 (bs,1H,OH), 7.24-7.55 (m,10H,CHAr). 13C NMR (CDCl3,100 MHz): δ 14.1,14.4, 20.6,32.9,39.9,41.7,42.9,46.2,58.9,72.3,91.6,124.9,125.7, 127.1,127.8,128.5,128.8,146.9,150.1,157.9,170.9. Anal. Calcd. for C25H31NO3: C,76.30; H,7.94; N,3.56. Found: C,76.41; H,7.85; N,3.61.
Ethyl 2-(butylamino)-4-(4-chlorophenyl)-4-hydroxy-6-phenylcyclohex- 1-enecarboxylate (4b): mp 118-119 ℃. IR (KBr, cm-1): υmax 3431.1,3276.8,3024.6,2932.1,1625.1,1592.5, 1452.1,1095.1. 1H NMR (CDCl3,400 MHz): δ 0.80 (t,3H, J = 7.1 Hz),1.01 (t,3H,J = 7.2 Hz),1.08 (m,2H),1.30 (m,2H), 2.27 (dd,1H,J = 13.7,11.0 Hz),2.38 (dd,1H,J = 13.7,6.8 Hz),2.46 (m,1H),2.75 (m,1H),3.12 (dd,1H,J = 11.0,6.8 Hz),3.53 (m,2H), 4.08 (q,2H,OCH2),6.59 (bs,1H,NH),7.50 (brs,1H,OH),7.24-7.55 (m,9H,CHAr). 13C NMR (CDCl3,100 MHz): δ 14.1,14.3,20.7,32.8, 39.9,41.5,42.9,45.9,59.1,71.9,91.6,125.7,126.7,127.1,128.6, 128.9,133.6,145.6,149.7,157.6,170.8. Anal. Calcd. for C25H30ClNO3: C,70.16; H,7.07; N,3.27. Found: C,70.11; H, 6.96; N,3.31.
Ethyl 2-amino-4-hydroxy-4,6-diphenylcyclohex-1-enecarboxylate (4c): mp 140-141 ℃. IR (KBr,cm-1): υmax 3473.1,3329.5, 2983.2,2924.5,1655.3,1609.6,1532.1,1360.3,1065.8,1H NMR (CDCl3,400 MHz): δ 0.9 (t,3H,J = 7.2 Hz),2.00 (dd,1H,J = 13.7, 11.2 Hz),2.27-2.35 (m,2H),2.41 (m,1H),3.01 (m,1H),3.73-4.00 (m,2H),6.20 (bs,2H),7.12-7.48 (m,10H),7.50 (bs,1H,OH),13C NMR (CDCl3,100 MHz): δ 14.1,39.9,45.4,46.7,59.4,72.6,94.9, 124.9,125.8,127.4,127.9,128.6,128.8,146.5,149.2,154.7, 170.2. Anal. Calcd. for C21H23NO3: C,74.75; H,6.87; N,4.15. Found: C,74.62; H,6.82; N,4.11.
Ethyl 2-amino-4-(4-chlorophenyl)-4-hydroxy-6-phenylcyclohex- 1-enecarboxylate (4d): mp 146-147 ℃. IR (KBr,cm-1): υmax 3489.5,3446.2,3310.3,2981.5,2945.6,1664.4,1612.3,1542.0, 1492.5,1366.8,1065.7; 1H NMR (CDCl3,400 MHz): δ 0.82 (t,3H, J = 7.2 Hz),1.97 (dd,1H,J = 13.7,11.2 Hz),2.25-2.41 (m,3H),2.99 (m,1H),3.75-4.00 (m,2H),6.20 (bs,2H),7.13-7.45 (m,9H),7.50 (bs,1H,OH); 13C NMR (CDCl3,100 MHz): δ 14.1,39.9,45.2,46.6, 59.3,72.4,94.8,125.9,126.6,127.3,128.6,128.9,133.6,145.2, 148.9,154.5,170.2. Anal. Calcd. for C21H22ClNO3: C,67.83; H,5.96; N,3.77. Found: C,67.71; H,5.91; N,3.82.
Ethyl 2-amino-6-(4-fluorophenyl)-4-hydroxy-4-phenylcyclohex- 1-enecarboxylate (4e): mp 168-171 ℃. IR (KBr,cm-1): υmax 3485.5,3447.2,3310.3,1628.5,1594.5,1449.0,1H NMR (CDCl3, 400 MHz): δ 0.9 (t,3H,J = 7.2 Hz),2.21(m,1H),2.36(m,2H),2.43(m, 1H),3.11(m,1H),3.75-4.00 (q,2H,J = 7.2 Hz),6.22 (bs,2H),6.93 (bs, 1H,OH),7.21-7.87 (m,9H); 13CNMR (CDCl3,100 MHz): δ 14.2,39.9, 45.6,46.8,59.6,72.8,94.9,124.9,125.9,127.5,127.9,128.7,128.9, 146.5,149.4,154.9,170.3. Anal. Calcd. for C21H22FNO3: C,70.97; H, 6.24; N,3.94; Found: C,70.91; H,6.15; N,3.89.
Ethyl 2-(butylamino)-6-(4-fluorophenyl)-4-hydroxy-4-phenylcyclohex- 1-enecarboxylate (4f): mp 126-129 ℃. IR (KBr, cm-1): υmax 3430.0,3276.5,3022.4,2931.1,1623.2,1593.5, 1094.2; 1H NMR (CDCl3,400 MHz): δ 0.69 (t,3H,J = 7.0 Hz),1.01 (t,3H,J = 7.2 Hz),1.09 (m,2H),1.43 (m,2H),2.30 (dd,1H,J = 13.7, 11.0 Hz),2.38 (dd,1H,J = 13.7,6.8 Hz),2.46 (m,1H),2.77 (m,1H), 3.12 (dd,1H,J = 11.0,6.8 Hz),3.20 (m,2H),4.04 (q,2H,J = 7.0 Hz, OCH2),6.59 (bs,1H,NH),7.40 (bs,1H,OH),7.24-7.49 (m,9 H, CHAr). 13C NMR (CDCl3,100 MHz): δ 14.0,14.2,20.5,32.6,39.9, 41.4,42.9,45.9,59.2,71.9,91.8,125.8,126.6,127.1,128.5,128.7, 133.6,145.5,149.5,157.4,170.5. Anal. Calcd. for C25H30FNO3: C, 72.97; H,7.35; N,3.40; Found: C,72.89; H,7.29; N,3.31.
2.4. Reusability of catalystThe recovered catalyst from the experiment was washed by acetone (3 × 6 mL). Then,it was dried in an oven at 100 ℃ and used in the synthesis of 2-aminocyclohex-1-ene-1-carboxylic esters. Then the catalyst was recycled for five times.
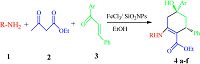
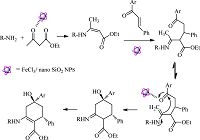
3. Results and discussionIn this article,we report a simple,mild and effective procedure for the one-pot synthesis of 2-aminocyclohex-1-ene-1-carboxylic esters using FeCl3/nano-SiO2 as a recyclable catalyst. This catalyst is safe,easy to handle and environmentally favorable. Initially,we carried out the MCR between butyl amine,ethyl acetoacetate and chalcone at room temperature as a model reaction in the presence of different catalysts. We tested several catalysts for this multicomponent synthesis. The model reaction was carried out in the presence of various catalysts such as FeCl3,nano SiO2,and FeCl3/ SiO2 nanoparticles. When the reaction was carried out using FeCl3, nano SiO2,and FeCl3/SiO2 as the catalyst,the product could be obtained in moderate to good yield. From the results,it is obvious that FeCl3/SiO2 NPs is the best catalyst among those examined which are reported in Table 1. When 0.3,0.6,and 0.9 mol% of FeCl3/ SiO2 nanoparticles were used,the yields were 73%,78% and 78%, respectively. Therefore,performing the reaction with a higher catalyst loading (0.9 mol%) had no noteworthy effect on yield. Nano silica supported ferric chloride is an effective catalyst in terms of reaction times and yields of product,because of the supported nano-SiO2 increase contact surface of materials. The SEM image of FeCl3/nano-SiO2 is shown Fig. 1. The SEM image shows particles with diameters in the range of nanometers. As shown in Fig. 1 the particle size of the FeCl3-SiO2 (np) has been found to be 45-50 nm. The reaction works well for different chalcone and primary amines (Table 2). The mechanism of these domino reactions is proposed in Scheme 2. Also,in the present reaction FeCl3-SiO2 NPs may play as Lewis solid acids. In this mechanism the FeCl3-SiO2 NPs act as Lewis solid acid and activate the C=O group for better reaction with nucleophiles. The mechanism has been supported by literature [17, 18, 19]. The increased surface area due to small particle size increased reactivity. The reaction proceeded with complete selectivity in favor of the diastereoisomer having a cis-arrangement for the aryl substituents at C-4 and C-6,with both substituents placed in an equatorial position [20]. The 1H NMR spectra in DMSO-d6 showed diastereotopic protons around δ 2.25-3.50. These diastereotopic protons give different signals,couple the protons on C-3 with each other and the protons on C-5 with C-6 (with different coupling constants),and couple with each other.
|
|
Table 1 Optimization of reaction condition using different catalysts using FeCl3/SiO2 NPs (0.6 mol%).a |

|
Download:
|
| Fig. 1.SEM images of FeCl3 nano-SiO2. | |
|
|
Table 2 Synthesis of 2-aminocyclohex-1-ene-1-carboxylic esters at room temperature in ethanol. |
|
Download:
|
| Scheme 2.Proposed reaction pathway for the synthesis of 2-aminocyclohex-1-ene-1-carboxylic esters using FeCl3/SiO2 NPs. | |
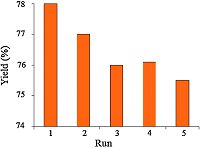
Wealso investigatedrecycling of the FeCl3-SiO2NPs as catalyst in ethanol. The results showed that FeCl3-SiO2 NPs can be recycled several times without noticeable loss of catalytic activity (yields 75%-78%) (Fig. 2). The leaching of the Fe from the FeCl3/SiO2 NPs nanoparticleswas investigated. After completion of the reaction,the supernatant was collected and tested for Fe by inductively coupled plasma-atomic emission spectroscopy (ICP-AES). The leaching of Fe in five continuous runs was found to be ≤0.6 ppm. We believe that, this is also the possible reason for the extreme stability of the FeCl3/ SiO2 NPs nano-particles presented herein.
|
Download:
|
| Fig. 2.Recycling of FeCl3 nano-SiO2 catalyst for the preparation of 4a. | |
In conclusion,we have developed a straightforward and efficient approach to synthesis of 2-aminocyclohex-1-ene-1- carboxylic esters by an easy one-pot three-component reaction of primary amines,ethyl acetoacetate and chalcones in ethanol using FeCl3-SiO2 NPs. The present methodology offers several advantages,such as good yields,room temperature,recyclable catalyst with a very easy operation and little catalyst loading.
AcknowledgmentThe authors acknowledge a reviewer who provided helpful insights. The authors are grateful to Islamic Azad University,Qom Branch.
| [1] | K.L. Kozielski, S.Y. Tzeng, J.J. Green, A bioreducible linear poly(β-amino ester) for siRNA delivery, Chem. Comm. 49 (2013) 5319-5321. |
| [2] | G.T. Zugates, N.C. Tedford, A. Zumbuehl, et al., Gene delivery properties of endmodified poly(β-amino ester)s, Bioconj. Chem. 18 (2007) 1887-1896. |
| [3] | K.R. Guertin, C.J. Gardner, S.I. Klein, et al., Optimization of the β-aminoester class of factor Xa inhibitors. Part 2: identification of FXV673 as a potent and selective inhibitor with excellent in vivo anticoagulant activity, Bioorg. Med. Chem. Lett. 12 (2002) 1671-1674. |
| [4] | J. Safaei-Ghomi, R. Teymuri, H. Shahbazi-Alavi, et al., SnCl2/nano SiO2: a green and reusable heterogeneous catalyst for the synthesis of polyfunctionalized 4Hpyrans, Chin. Chem. Lett. 24 (2013) 921-925. |
| [5] | J.P. Wan, Y. Liu, Recent advances in new multicomponent synthesis of structurally diversified 1,4-dihydropyridines, RSC Adv. 2 (2012) 9763-9777. |
| [6] | B. Jiang, T. Rajale, W. Wever, et al., Multicomponent reactions for the synthesis of heterocycles, Chem. Asian J. 5 (2010) 2318-2335. |
| [7] | F. Yu, R. Huang, H. Ni, et al., Three-component stereoselective synthesis of spirooxindole derivatives, Green Chem. 15 (2013) 453-462. |
| [8] | A. Bhunia, T. Kaicharla, D. Porwal, et al., Multicomponent reactions involving phosphines, arynes and aldehydes, Chem. Commun. 50 (2014) 11389-11392. |
| [9] | J. Safaei-Ghomi, H. Shahbazi-Alavi, E. Heidari-Baghbahadorani, SnO nanoparticles as an efficient catalyst for the one-pot synthesis of chromeno[2,3-b]pyridines and 2-amino-3,5-dicyano-6-sulfanyl pyridines, RSC Adv. 4 (2014) 50668-50677. |
| [10] | X.H. Zhanga, J.F. Yanb, L. Fana, et al., Synthesis and antidiabetic activity of β-acetamido ketones, Acta Pharm. Sin. B 2 (2011) 100-105. |
| [11] | V.M. Joshi, R.L. Magar, P.B. Throat, et al., Novel one-pot synthesis of 4H-chromene derivatives using amino functionalized silica gel catalyst, Chin. Chem. Lett. 25 (2014) 455-458. |
| [12] | M. Kassaee, R. Mohammadi, H. Masrouri, F. Movahedi, Nano TiO2 as a heterogeneous catalyst in an efficient one-pot three-component Mannich synthesis of β-aminocarbonyls, Chin. Chem. Lett. 22 (2011) 1203-1206. |
| [13] | F. Alonso, Y. Moglie, G. Radivoy, et al., Multicomponent click synthesis of 1,2,3-triazoles from epoxides in water catalyzed by copper nanoparticles on activated carbon, J. Org. Chem. 76 (2011) 8394-8405. |
| [14] | N. Koukabi, E. Kolvari, A. Khazaei, et al., Hantzsch reaction on free nano-Fe2O3-catalyst: excellent reactivity combined with facile catalyst recovery and recyclability, Chem. Commun. 47 (2011) 9230-9232. |
| [15] | K. Chanda, S. Rej, M.H. Huang, Facet-dependent catalytic activity of Cu2O nanocrystals in the one-pot synthesis of 1,2,3-triazoles by multicomponent click reactions, Chem. Eur. J. 19 (2013) 16036-16043. |
| [16] | M.B. Gawande, A. Velhinho, I.D. Nogueira, et al., A facile synthesis of cysteine-ferrite magnetic nanoparticles for application in multicomponent reactions -a sustainable protocol, RSC Adv. 2 (2012) 6144-6149. |
| [17] | L. Abahmane, J.M. Köhler, G.A. Groß, Gold-nanoparticle-catalyzed synthesis of propargylamines: the traditional A3-multicomponent reaction performed as a two-step flow process, Chem. Eur. J. 17 (2011) 3005-3010. |
| [18] | C.W. Lim, I.S. Lee, Magnetically recyclable nanocatalyst systems for the organic reactions, Nano Today 5 (2010) 412-434. |
| [19] | A.R. Kiasat, J. Davarpanah, Fe3O4@silica sulfuric acid nanoparticles: an efficient reusable nanomagnetic catalyst as potent solid acid for one-pot solvent-free synthesis of indazolo[21-b]phthalazine-triones and pyrazolo[1,2-b]phthalazinediones, J. Mol. Catal. A: Chem. 373 (2013) 46-54. |
| [20] | A. Mobinikhaledi, H. Moghanian, S. Pakdel, Microwave-assisted efficient synthesis of azlactone derivatives using 2-aminopyridine-functionalized sphere SiO2 nanoparticles as a reusable heterogeneous catalyst, Chin. Chem. Lett. 26 (2015) 557-563. |
| [21] | Y.G. Sun, T.T. Truong, Y.Z. Liu, Y.X. Hu, Encapsulation of superparamagnetic Fe3O4@SiO2 core/shell nanoparticles in MnO2 microflowers with high surface areas, Chin. Chem. Lett. 26 (2015) 233-237. |
| [22] | J. Safaei-Ghomi, R. Teymuri, H. Shahbazi-Alavi, A. Ziarati, SnCl2/nano SiO2: a green and reusable heterogeneous catalyst for the synthesis of polyfunctionalized 4Hpyrans, Chin. Chem. Lett. 24 (2013) 921-925. |
| [23] | J. Safaei-Ghomi, A. Ziarati, S. Zahedi, Silica (NPs) supported Fe (III) as a reusable heterogeneous catalyst for the one-pot synthesis of 1, 4-dihydropyridines under mild conditions, J. Chem. Sci. 124 (2012) 933-939. |
| [24] | J. Safaei-Ghomi, M.A. Ghasemzadeh, S. Zahedi, FeCl3 nano SiO2: an efficient heterogeneous nano catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]-xanthenes and 1,8-dioxo-octahydro-xanthenes under solvent-free conditions, S. Afr. J. Chem. 65 (2012) 191-195. |
| [25] | J. Safaei-Ghomi, S. Zahedi, M.A. Ghasemzadeh, Nano silica supported ferric chloride as a green and efficient catalyst for one pot synthesis of 12-dihydro-1-arylnaphtho[1,2-e][1,3]oxazine-3-ones, Iran J. Catal. 2 (2012) 27-30. |
| [26] | V. Sridharan, J.C. Menendez, Two-step stereocontrolled synthesis of densely functionalized cyclic β-aminoesters containing four stereocenters, based on a new cerium(IV) ammonium nitrate catalyzed sequential three-component reaction, Org. Lett. 10 (2008) 4303-4306. |